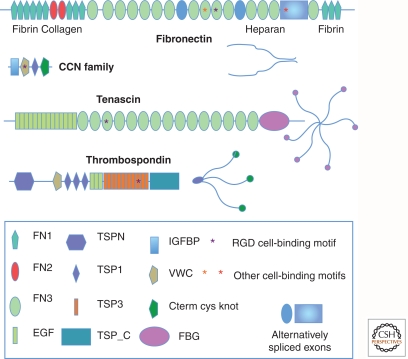
Figure 2.
Examples of characteristic ECM glycoprotein structures. Note the multidomain structure of these ECM glycoproteins. Each domain is typically encoded by a single exon or a small set of exons. This has allowed shuffling of domains into different combinations during evolution. Individual domains are specialized for binding different proteins, as indicated for fibronectin. Some domains are alternatively spliced, as noted for fibronectin, and is also true for tenascin (not shown). Cell-binding motifs such as RGD and LGV are indicated by asterisks. Fibronectin dimerizes through disulfide bonding at the carboxyl terminus, whereas thrombospondin and tenascin form trimers and hexamers, respectively, through coiled coil domains and disulfide bonds near the amino terminus. The appearances of the intact protein multimers (as would be seen by electron microscopy) are diagrammed. Note that growth factor-binding domains (IGFBP, VWC, and others) are included in many ECM proteins. CYR61 is shown as a representative member of the CCN family (see Table 2), small ECM proteins that contain integrin-binding motifs and growth-factor-binding domains (IGFBP and VWC) and are known to regulate growth factor functions (Chen and Lau 2009) as are the larger proteins shown.