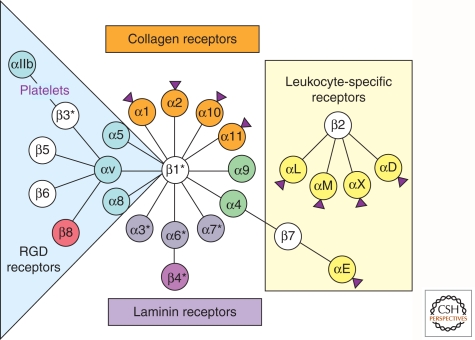
Figure 4.
Integrin receptors for ECM proteins. The diversity of integrin subunits and their interactions. Shown are the mammalian integrins, separated by color coding into subsets of closely related subunits. The RGD- binding (blue) and laminin-binding (purple) subclasses are evolutionarily very ancient and found in all metazoan phyla, but they have diverged into clades in the vertebrate lineage. The α4/α9 clade (green) is vertebrate-specific. Two subclasses of chordate α subunits have inserted I domains (purple arrowheads); they include collagen-specific integrins (orange) and a set of α subunits confined to leukocytes (yellow). Some subunits show alternatively spliced isoforms (*). The leukocyte integrins bind predominantly to cell-surface counter-receptors, whereas integrins containing either the β1 or αv subunits bind predominantly to extracellular matrix (ECM) proteins, although within each class there are exceptions to these generalizations and it is worth noting that most integrins are capable of binding multiple ligands, and there are many others beyond those shown here (Humphries et al. 2006). Many, if not all, αv integrins are also capable of activating TGF-β. Most β subunits are highly related (white) and bind to talin and related proteins (Campbell and Humphries 2011; Wickström et al. 2011), whereas the β4 subunit instead binds to intermediate filaments through specific linker proteins and the β8 subunit binds to band 4.1 proteins instead of to talin. (figure modified from Hynes 2002).