Table 1.
Extracellular matrix proteoglycans
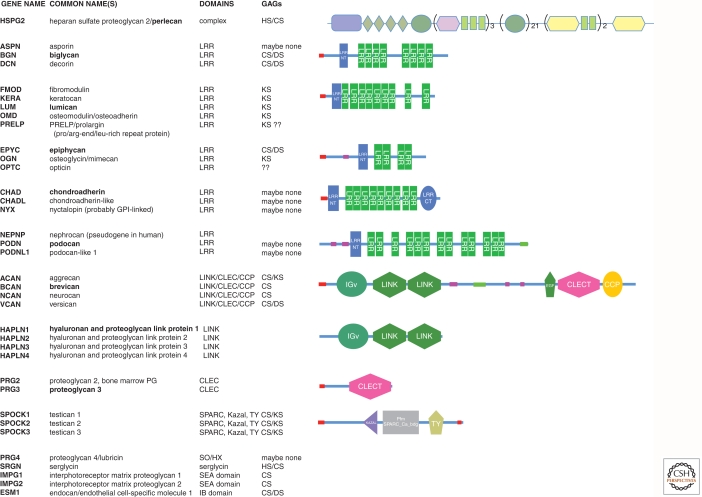 |
Table lists known ECM proteoglycans and shows representative domain structures.
There are five subfamilies of small leucine-rich repeat (LRR) proteoglycans based on sequence homologies (Merline et al. 2009; Schaefer and Schaefer 2010)—A representative structure is shown for one member (bold) from each subfamily. LRR repeat regions in other proteins are involved in protein–protein interactions.
The hyalectans (aggrecan, brevican, neurocan, and versican) all share a similar structure with Ig-LINK-LINK at their amino terminus and EGF(1 or 2)-CLEC-CCP at their carboxyl terminus with variable lengths of sequence between these two sets of domains. The central intervening sequence bears the GAG side chains and is almost 2000 amino acids long in aggrecan, and in versican it varies from a few to almost 2700 amino acids through alternative splicing. The LINK domains in both hyalectans and in the four link proteins bind to hyaluronan with nanomolar affinity and the EGF-CLEC-CCP cluster, which is similar to that in selectins, binds carbohydrates.
The domain structures were extracted from the SMART database (http://smart.embl-heidelberg.de). The domain structure of perlecan, the major proteoglycan of basement membranes, includes multiple domains in N—C order (SEA, LDLa, Ig, LamB, EGF, Ig, LamG, EGF). The glycosaminoglycan (GAG) content of each proteoglycan is based on the reviews cited above (HS, heparan sulfate; CS, chondroitin sulfate; DS, dermatan sulfate; KS, keratan sulfate).
