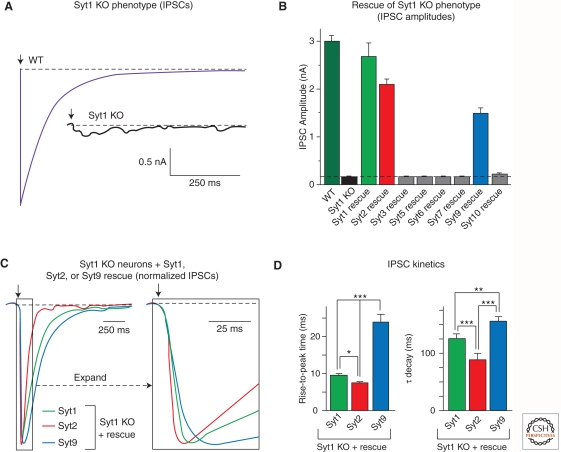
Figure 3.
Syt1, Syt2, and Syt9 function as synaptic Ca2+ sensors for neurotransmitter release. (A) Deletion of Syt1 in cortical neurons blocks fast synchronous neurotransmitter release. Panels depict representative inhibitory postsynaptic currents (IPSCs) monitored in cortical neurons cultured from littermate wild-type (WT) and Syt1 knockout (Syt1 KO) neurons (arrow = action potential). These data and those shown in panels B–D were modified from Xu et al. (2007). (B) Screen of all Ca2+-binding synaptotagmin isoforms for their ability to rescue the loss of fast neurotransmitter release in Syt1 KO neurons. Data shown are means IPSC amplitudes (± s.e.m.); note that only Syt1, 2, and 9 are capable of rescue. (C) Syt1, Syt2, and Syt9 mediate Ca2+ triggering of release with distinct kinetics. Data shown are representative IPSCs monitored in Syt1 KO neurons expressing Syt1, 2, or 9. IPSCs were normalized for the maximal amplitude. The traces on the right display an expanded part of the overall IPSCs depicted on the left. (D) Quantitation of the kinetics of the increase in the IPSC (left, as mean rise time) and the decay of the IPSC (right, as mean time constant of the IPSC decay) triggered by Ca2+ binding to Syt1, 2, or 9. Note that IPSCs mediated by Syt2 exhibit the fastest rise and decay kinetics, whereas IPSCs mediated by Syt9 are twofold slower. All error bars indicate s.e.m.; asterisks indicate statistically significant differences as assessed by Student’s t-test (*p < 0.05; **p < 0.01; ***p < 0.001).