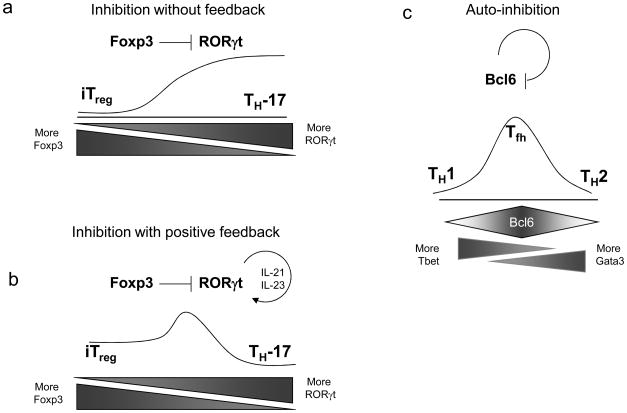
Figure 4.
Possible transcription factor interactions regulating intermediate CD4+ T cell transitions. (a) Hypothetical circuit in which Foxp3 inhibits the activity of RORγt, without a reciprocal inhibition or feedback from RORγt. This circuit might favor iTreg development over TH17 development, making iTreg more stable. Without continuous stimulus by IL-6 to induce RORγt, Foxp3 might tend to repress TH17 development. (b) This hypothetical TH17 state is stabilized despite inhibition of RORγt by Foxp3 due to a known feedback loop, in which RORγt induces expression of IL-21, which acts in an autocrine manner to further induce STAT3 and stimulate RORγt expression. This feedback stabilizes the TH17 state relative to iTreg cells. (c) The transcriptional inhibitor Bcl-6 inhibits its own transcription. Such a mechanism could destabilize the TFH phenotype, consistent with the fact that in vitro cultures of TFH phenotype cells have not been reported as stable, in contrast to the robust stability of TH1 and TH2 cells.