Figure 1. Characterization of IMPs.
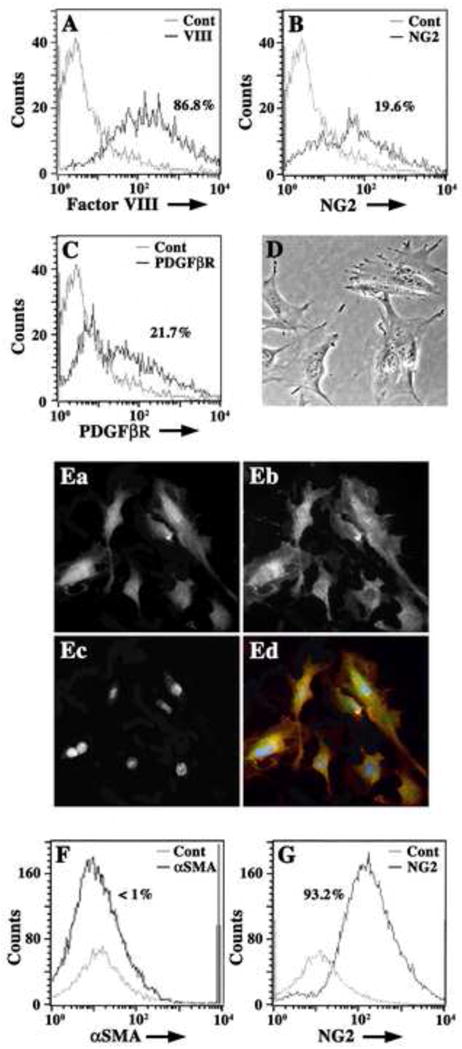
Freshly isolated Immortomouse® capillaries were enzymatically disrupted and single cell suspensions were analyzed by flow cytometry for the expression of pericyte (NG2, PDGFβR) and EC markers (Factor VIII) (A–C). In (A), a histogram overlay of isotype negative control antibody (grey line) and Factor VIII (black line) show that cell suspensions are predominately Factor VIII+ (86.8%) ECs. In (B), histogram overlays of negative isotype controls (grey line) and NG2+ cells (black line) show that cell suspensions also contain approximately 20% NG2+ cells (19.6%). In (C), histogram overlays of isotype negative controls (grey line) and PDGFβR+ cells (black line) show that 21.7% of cells express this pericyte marker. Cell suspensions plated at 5 × 105 cells/ml on plastic Petri dishes were incubated in standard culture medium for six hours at 37°C before non-adherent cells were washed off vigorously. Adherent cells are morphologically similar to pericytes (D) and are NG2+ (Ea; Ed, green) and PDGFβR+ (Eb; Ed, red) by dual immunocytochemistry. Nuclei are labeled with DAPI (Ec; Ed, blue). Adherent cells (for 48 hours) were suspended using RPMI-1640-EDTA and analyzed by flow cytometry for expression of αSMA and NG2. Histogram overlays from FACS analysis in (F) show negative control antibody (grey line) and αSMA+ cells (black line) and indicate that adherent cells are less than 1% αSMA+. Histogram overlays in (G) show control isotype antibody staining (grey line) and NG2 antibody staining (black line) and indicate that adherent cell suspensions are 93.2% NG2+. Histogram data are presented as cell number versus log fluorescence intensity (FITC) of individual cell markers.
