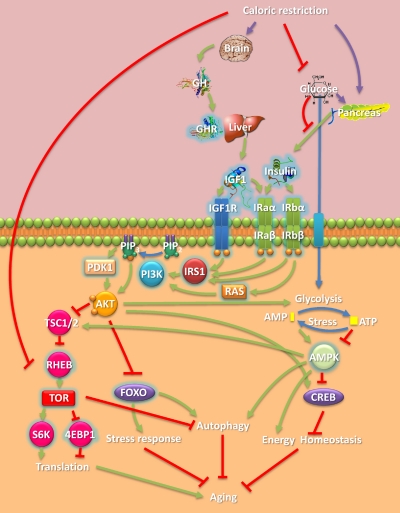
Fig. 1.
Overview of CR-associated signaling and some of its key players. CR acts on the hypothalamus, which controls the secretion of GH from the pituitary. CR also lowers glucose levels and diminishes secretion of insulin from the pancreas. GH acting on the liver causes the release to the plasma of IGF1. IGF1 binds to IGF1R or insulin receptor a (IRaα-IRaβ) and triggers its autophosphorylation, which in turn serves as an anchor for recruiting various downstream effectors. Phosphotidylinositol-3-kinase (PI3K) is either activated via direct interaction with, for example, the insulin-like substrate 1 (IRS1) or RAS. PI3K catalyzes the phosphorylation of phosphatidyl-inositols, such as the conversion of phosphatidyl inositol 4,5-bisphosphate (PIP2) to phosphatidyl inositol 3,4,5-trisphosphate (PIP3). PIP3 serves as a binding site for phosphoinositide-dependent protein kinase (PDK1), which activates the serine/threonine protein kinase AKT. AKT phosphorylates tuberous sclerosis protein 1 and 2 (TSC1/2) as well as FOXO transcription factors and cAMP response element-binding (CREB). TSC1/2 phosphorylation by AKT inhibits the Ras homolog enriched in brain (RHEB). RHEB stimulates the phosphorylation of the ribosomal protein S6 kinase (S6K1) and 4E-BP1 through activation of TOR. TOR's activation of S6K1 and inhibition of 4E-BP1 enhances translation. FOXO phosphorylation prevents its nuclear translocation and activation of its stress response target genes. Various cellular forms of stress and AKT activity lead to the depletion of ATP and elevation of AMP. AMPK positively regulates TSC2 and FOXO, negatively regulates CREB, and enforces energy homeostasis. FOXO and AMPK promote autophagy, whereas TOR suppresses it. Under CR, GH levels decline and therefore, via this pathway, TOR signaling decreases (CR also suppresses TOR signaling through other mechanisms) whereas AMPK and some FOXO factors are activated, in turn decreasing translation while increasing stress responses and autophagy, which seem to be some of the mechanisms by which CR retards aging. Human homologs of genes directly linked to CR life-extending effects in model organisms are highlighted with a blue halo.