Abstract
Background
Studies have suggested that patients with schizophrenia are impaired at recognizing emotions. Recently, it has been shown that the neuropeptide oxytocin can have beneficial effects on social behaviors.
Method
To examine emotion recognition deficits in patients and see whether oxytocin could improve these deficits, we carried out two experiments. In the first experiment we recruited 30 patients with schizophrenia and 29 age- and IQ-matched control subjects, and gave them an emotion recognition task. Following this, we carried out a second experiment in which we recruited 21 patients with schizophrenia for a double-blind, placebo-controlled cross-over study of the effects of oxytocin on the same emotion recognition task.
Results
In the first experiment we found that patients with schizophrenia had a deficit relative to controls in recognizing emotions. In the second experiment we found that administration of oxytocin improved the ability of patients to recognize emotions. The improvement was consistent and occurred for most emotions, and was present whether patients were identifying morphed or non-morphed faces.
Conclusions
These data add to a growing literature showing beneficial effects of oxytocin on social–behavioral tasks, as well as clinical symptoms.
Keywords: Emotion, faces, oxytocin, schizophrenia, social
Introduction
Impairments in social functioning are common in schizophrenia. Numerous studies have shown impaired perception of emotional expressions in schizophrenia (Edwards et al. 2002; Tremeau, 2006) and unaffected first-degree relatives also seem to be impaired, albeit to a lesser extent (Bediou et al. 2007). Further, difficulties in classifying emotions have been shown to contribute to social-skill deficits (Morris et al. 2009).
Recent results have suggested that the nonapeptide oxytocin can improve emotion recognition in patients with autism spectrum disorders (ASDs; Guastella et al. 2010). Related studies in this patient group have also shown that oxytocin increases social interactions. Specifically, when patients with ASD played a game in which they could choose to throw a ball to one of three players, treatment with oxytocin increased interactions with players that were more likely to throw the ball back to the patient (Andari et al. 2010). In the same study oxytocin treatment also increased ratings of trust and preference for the most interactive player. Additional studies have also shown that oxytocin increases saccades to the eye region when patients with ASD (Andari et al. 2010) or healthy subjects (Guastella et al. 2008a) viewed pictures of faces. Imaging data has also shown that the increased saccades to the eye region on oxytocin are likely driven by amygdala–brain stem interactions (Gamer et al. 2010).
In the current experiments we hypothesized that: (1) patients with schizophrenia would have a deficit relative to a control group on recognizing emotions; and (2) oxytocin could ameliorate some of this deficit. To examine these hypotheses we carried out two experiments. In the first, a group of patients with schizophrenia was compared with a group of control participants on an emotion recognition task and we found that patients had a deficit in emotion recognition. In the second experiment the effects of oxytocin on the performance of the same emotion recognition task were examined in a group of patients with schizophrenia. We found that administration of oxytocin significantly increased the ability of the patients to identify the emotions in the task.
Method
Patients
All subjects were recruited from the out-patient department of South London and Maudsley NHS Trust (London, UK). Two groups of patients and one group of controls were recruited for separate experiments. The first experiment compared patients with controls on an emotion discrimination task, and the second experiment examined the effects of oxytocin in a patient group (partially overlapping with the first patient group) on the same task. Most aspects of the protocol were the same for the two experiments. Specific details are given for the differences. For experiment 1, in which patients were compared with a matched control group on the emotion discrimination task, we recruited 30 individuals (of which 24 were male) who met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for schizophrenia (Table 1). In addition to their anti-psychotics (Table 1), one of these individuals was taking lithium and one zopiclone, which is a sleep aid. For experiment 2, in which patients with schizophrenia were studied on oxytocin and placebo on the emotion discrimination task, we recruited 21 individuals (all were male) who met DSM-IV criteria for schizophrenia (Table 2). In addition to their anti-psychotics, two of these patients were additionally on selective serotonin reuptake inhibitors (SSRIs), one on a benzodiazepine, one on an anti-cholinergic and one on a vesicular monoamine transport (VMAT) inhibitor, which reduces available dopamine stores. Chlorpromazine equivalent units were calculated according to published tables (Woods, 2003) and the British National Formulary (BNF 54; British Medical Association & the Royal Pharmaceutical Society of Great Britain). All patients were assessed by an experienced clinician who determined whether they met the DSM-IV criteria for schizophrenia and made the diagnosis. Patients also underwent a Positive and Negative Symptom Scale (PANSS) interview (Kay et al. 1987) on the first day of testing in each experiment. The PANSS is a clinical interview which assesses the patients on seven aspects of positive symptoms, seven aspects of negative symptoms and 16 aspects of general symptomatology. All participants in the oxytocin study were male because oxytocin can induce uterine contractions in pregnant females and therefore it poses increased risks. Of the participants in the oxytocin group, 11 had participated in the original study comparing controls and patients. The time between the two experiments in this group of eleven patients was on average 21.6 months, with a minimum of 10 months. A t test on overall performance in the oxytocin experiment, between the group of patients that had participated in the original study and the patients newly recruited for the oxytocin study, showed no difference (p=0.774). We also compared performance on the task in the group that was tested twice, by correlating performance across conditions, within subject. For this we used only the placebo data from the second experiment. The correlation between sessions was 0.57 (s.d. 0.35). Most of the correlations were relatively high. However, two of the patients had low correlations (−0.01 and −0.08).
Table 1.
Participant demographic information for comparison between patients and controls
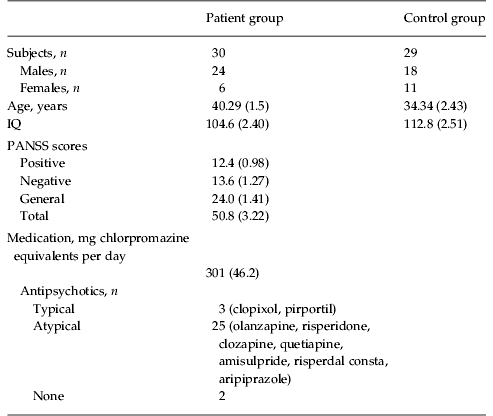
Data are given as mean (standard error of the mean) or as number of subjects.
IQ, Intelligence quotient; PANSS, Positive and Negative Symptom Scale.
Table 2.
Participant demographic information for oxytocin study
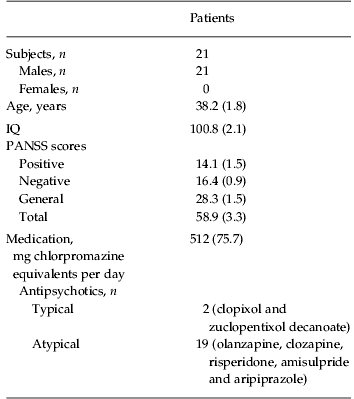
Data are given as mean (standard error of the mean) or as number of subjects.
IQ, Intelligence quotient; PANSS, Positive and Negative Symptom Scale.
All subjects provided written consent. Standard consent and safety reporting procedures were followed, and ethical approval was obtained from the South London and Maudsley, Institute of Psychiatry, King's College London research ethics committee. Patients were stable on treatment with antipsychotic medication (see Tables 1 and 2 for type of medication). Those with dual diagnoses and drug and alcohol problems were excluded from the study. In the oxytocin study, participants were also excluded if they had high blood pressure (greater than 140/90 mmHg), heart problems or any major non-psychiatric illness or hospitalization in the last year.
Control group
For the study which compared patients with controls, a group of 29 control participants was recruited (Table 1). These were age, gender and IQ matched to the patient group. IQ was estimated from errors in the National Adult Reading Test. Inclusion criteria stipulated no psychiatric history and no history of drug or alcohol problems.
Oxytocin administration
The oxytocin study was carried out using a double-blind placebo-controlled cross-over design, so neither the individual administering the drug nor the participant knew if they were receiving the drug or placebo. Each participant completed two sessions of testing separated by 7 or 8 days (mean=7.33, s.e.m.=0.10 days). All recruited participants performed the task in both sessions and none of the participants dropped out. The procedure was the same for each session. Upon arrival, the participant self-administered a nasal spray consisting of either 24 IU oxytocin (Syntocinon, Novartis) or saline placebo. This is similar to the dose which has been used in previous studies which have shown effects (Kirsch et al. 2005; Guastella et al. 2008b; Savaskan et al. 2008; Rimmele et al. 2009; Evans et al. 2010). Each participant received both oxytocin and placebo and the order in which they received them was randomized. Of 21 subjects, 11 received placebo first and 10 received oxytocin first. Of the 11 participants that had been involved in the original experiment comparing controls and patients on emotion recognition, six received placebo first and five received oxytocin first.
Following administration, behavioral testing commenced after a 50-min delay. It has been shown that vasopressin, which is closely related to oxytocin, reaches peak effects in 30–50 min when administered transnasally (Born et al. 2002), and a 50-min delay between drug administration and the start of testing has been used in previous studies with oxytocin (Kirsch et al. 2005; Domes et al. 2007b; Evans et al. 2010; Fischer-Shofty et al. 2010).
To examine non-specific effects of the drug on mood, participants completed a questionnaire just before testing began, 50 min after drug delivery, on both the drug and placebo visit. The questionnaire used was the Brief Mood Inventory Scale (Mayer & Gaschke, 1988). Participants rated 16 dimensions of mood on a scale of 1 to 4, followed by overall mood on a scale of −10 to +10. There was no effect of the drug on the patients' pleasant/unpleasant ratings score [t(20)=1.24, p=0.228] or on overall mood [t(20)=1.18, p=0.253], consistent with several previous studies in healthy participants (Kirsch et al. 2005; Di Simplicio et al. 2009; Evans et al. 2010; Fischer-Shofty et al. 2010; Marsh et al. 2010). Safety reporting procedures were in accordance with local research and development guidelines. Participants were monitored closely following spray delivery. No adverse events occurred.
Task
Participants in both experiments carried out the hexagon emotion discrimination task, used to characterize emotion recognition (Calder et al. 1996). They received instructions on-screen prior to the task. The instructions were as follows. ‘You will see a face presented on the screen. You are to indicate which word best describes the emotion in the face. Please press keys 1 to 6 to choose the emotions.’ On each trial, a face was presented for 1000 ms. This was followed by a choice screen which presented the options with a number next to each emotion name. Participants used the keyboard to indicate their choice. The faces were drawn from the Ekman series (Ekman & Friesen, 1971). Both unmorphed and morphed (70%/30%) faces were used. There were a total of 192 trials, of which half were morphed. Four different identities (IDs) were used [two male (IDs 2 and 10) and two female (IDs 9 and 11) from the Ekman set] and each image was shown four times, not sequentially. Stimulus order was randomized across all conditions including morphed and unmorphed. Morphs were between the following pairs of emotions: happiness–surprise; surprise–fear; fear–sadness; sadness–disgust; disgust–anger; anger–happiness. Morphs between these emotions are used as these are the emotions which are most often confused when emotions in unmorphed faces are identified (Calder et al. 1996). The morphs were generated using Abrosoft Fantamorph software (Abrosoft, USA). To morph the images, corresponding points are first defined on the two images between which the morphs would be produced. The points were located on the eyes, nose, mouth, chin and forehead. The software then stretches the images, as if they were printed on a sheet of rubber, such that points on the morphed image lie between the points on each of the unmorphed images, with the distance given by the morph fraction. For example, for a 70% fearful/30% sad morph, the points would be stretched so they would lie 70% of the way to the fearful face, from the sad face, and correspondingly 30% of the way to the sad face, from the fearful face. The pixel intensity values were then blended between the two stretched faces, using the appropriate weight, for example, a 70% weight on the fearful and a 30% weight on the sad pixel intensities. Additional details of the procedure have been given previously (Calder et al. 1996).
Data analysis
Trials involving morphed and unmorphed faces were analysed separately. Percentage correct was calculated for each of the six emotions presented, under the morphed and unmorphed conditions. For morphed trials, the majority emotion present was considered the correct choice. These values were then entered as the dependent variable in a three-way mixed-effects analysis of variance (ANOVA) with morph, emotion and drug as main effects for the oxytocin study, or morph, emotion and group as main effects for the control study. Thus, the effects on all emotions were assessed in an omnibus ANOVA. Order of drug versus placebo session was added as a factor in the analysis of the oxytocin data. ANOVA assumptions were checked by examining residuals for normality. Post-hoc t tests were not corrected for multiple comparisons. This allows for more direct comparison with other studies which may use fewer emotions.
Results
Experiment 1: comparison of patients and controls
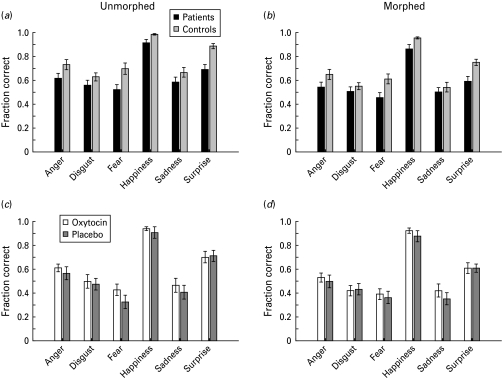
In experiment 1, when the patient group was compared with a matched control group, the patients were less accurate than controls in both morphed and unmorphed conditions and the performance of both groups was worse in the morphed condition (Fig. 1a, b). There were main effects of group [F(1, 57)=14.41, p<0.001], emotion [F(5, 285)=40.95, p<0.001] and morphing [F(1, 342)=125.25, p<0.001], but neither the emotion×group [F(1, 285)=1.36, p=0.239] nor the group×morphing [F(1, 342)=1.62, p=0.204] interaction was significant. Therefore, lowering the emotional intensity by using morphed stimuli did reduce performance, but there was no differential impairment between patients and controls. When individual emotions were compared separately between groups, there were significant differences (uncorrected t tests, p<0.05) in fear, happiness and surprise in both the morphed and unmorphed conditions (Table 3).
Fig. 1.
Fraction correct for each emotion. (a) Patients v. controls unmorphed faces. (b) Patients v. controls morphed faces. (c) Patients on v. off oxytocin unmorphed faces. (d) Patients on v. off oxytocin morphed faces. Data are means, with standard errors of the mean computed across participants represented by vertical bars.
Table 3.
Statistics on individual emotions for comparison of patients and controls
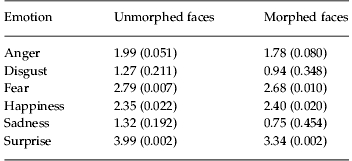
Data are given as t statistic (p).
To control for IQ-related performance effects, we examined correlations between IQ scores and overall accuracy on morphed and unmorphed trials combined. In patients there was no significant correlation [R(29)=0.185, p=0.327]. However in controls there was a significant correlation [R(28)=0.619, p<0.001]. IQ scores for all subjects were then entered in the ANOVA as a covariate to confirm that variation in IQ could not account for the group differences. The main effect of group was still present [F(1, 57)=8.88, p=0.006] with IQ-related variance removed. A similar analysis was performed to account for medication status. There was no significant correlation between medication status in chlorpromazine equivalent units and overall performance in the patient group [R(23)=0.067, p=0.757].
Experiment 2: effects of oxytocin in patients
Subsequent to the first experiment showing decreased performance in patients relative to matched controls, we carried out a separate experiment in a patient group to examine the effects of oxytocin on emotion discrimination. Analysis of the fraction correct data of each participant, comparing oxytocin with placebo, morphed with non-morphed trials for each emotion (Fig. 1c, d) showed main effects of drug [F(1, 264)=7.74, p=0.006], morph [F(1, 322)=22.31, p<0.001] and emotion [F(5, 100)=35.42, p<0.001]. There were no significant interactions (p>0.447). Although the overall performance was generally improved on oxytocin, none of the individual emotions showed a significant difference (t test, p<0.05), although fear showed a trend in the unmorphed condition (Table 4).
Table 4.
Statistics on individual emotions for effects of oxytocin in patients
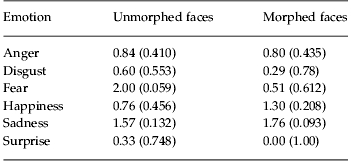
Data are given as t statistic (p).
As we only carried out PANSS interviews on the first day of testing, we could not examine changes in PANSS scores with the drug. However, we did correlate the improvement in emotion recognition with positive, negative and general PANSS scores, across subjects. None of the correlations was significant, however (p>0.10).
In the final analysis we compared the performance of the patients from the second experiment on oxytocin with the performance of the control group in the first experiment, to see the effect of oxytocin on the group difference. Performance of the patients on oxytocin was, however, still lower than the performance of the control group, as there was still a main effect of group [F(1, 48)=26.7, p<0.001]. The control group performed at 72% correct, the patient group off oxytocin in the second experiment at 54% correct and the patient group on oxytocin at 58% correct. Thus, oxytocin made up 22% of the difference in performance.
Discussion
In the first experiment, we found that patients with schizophrenia were less accurate at identifying emotions than a group of matched controls. This is consistent with several previous studies which have shown that patients with schizophrenia (Edwards et al. 2002; Kohler et al. 2003; Bediou et al. 2005; Tremeau, 2006; Derntl et al. 2009) and their first-degree relatives (Bediou et al. 2007) have a deficit in identifying emotions. Other studies have also suggested that this deficit may contribute to deficits in social skills (Morris et al. 2009). We did not find, in our study, that there were emotion×group or group×morphing interactions. Thus, patients were generally worse at recognizing emotions. Future studies could utilize a test of face recognition, for example, the Benton Facial Recognition Test (Benton et al. 1994) to examine whether the effects were specific to identifying emotions, or more general deficits in processing face information. Most of our patients were also on antipsychotic medication, and it is currently not clear the extent to which medication might contribute to deficits in emotion recognition. In the second experiment we found that administration of the nonapeptide oxytocin significantly improved the ability of patients with schizophrenia to identify the basic emotions. Emotion identification is a well-established issue that may have general consequences for social behavior in patients and oxytocin may help to ameliorate this deficit. Effects in our task were relatively modest, however, and the sample size was also relatively small. Additional experiments will help to substantiate these effects.
There has been considerable interest in the social effects of the neuropeptide oxytocin. Oxytocin is produced in the hypothalamus and it is released into the bloodstream where it mediates contractions of the uterus during parturition as well as milk release during breast feeding (Burbach et al. 2006). However, oxytocin is also released into the central nervous system (Veening et al. 2010), where it can bond to oxytocin receptors in various brain areas (Schorscher-Petcu et al. 2009). Work in rodents has shown important effects of oxytocin on pair bonding and maternal behaviors (Pedersen et al. 1982; Fahrbach et al. 1985; Williams et al. 1994; Cho et al. 1999; Lim & Young, 2006).
The effects of oxytocin on social behaviors in human subjects have also been examined recently. These studies have found that oxytocin can improve emotion identification in the reading the mind from the eyes task, in healthy human participants (Domes et al. 2007b). This improvement in performance may come from the fact that oxytocin increases saccades to the eye region (Guastella et al. 2008a), an effect mediated by interactions between the amygdala and brain stem regions involved in saccade generation (Gamer et al. 2010). Other work has shown that oxytocin can decrease aversion to angry faces in decision-making tasks (Evans et al. 2010), an effect which might be mediated by decreased amygdala responses to angry faces (Kirsch et al. 2005), although other work has shown that oxytocin decreases amygdala responses to other emotions as well (Domes et al. 2007a). Related to this, oxytocin has also been shown to decrease negative affective evaluation of faces that have been negatively conditioned with shock, an effect also mediated by the amygdala (Petrovic et al. 2008). One limitation of our second study utilizing oxytocin is that only male participants were tested, as is the case in most studies of oxytocin. This is an important limitation that should be examined in future studies as it has been shown that oxytocin affects processing of emotional faces differently in males and females (Domes et al. 2010).
Interest in oxytocin has also extended to clinical populations characterized by some degree of social deficits, including ASD and schizophrenia. Work in these groups has shown that oxytocin can improve emotion recognition deficits in patients with ASD (Guastella et al. 2010). It also increases the incidence with which patients with ASD choose to interact with highly social individuals in games, and increases ratings of trust and preference for these highly social individuals, by the participants with ASD (Andari et al. 2010). Thus, oxytocin appears to improve some basic social deficits in ASD. Although rodent studies have generally found that oxytocin's effects are specific to social function (Lim & Young, 2006), the extent to which oxytocin might improve general cognitive function, as opposed to specifically social function, is an open question in human studies. Modafinil, for example, which affects alertness, has been shown to improve the recognition of emotions (Scoriels et al. 2011).
Interest in oxytocin in schizophrenia is just beginning to emerge. Recent studies have shown improvements in clinical symptoms, including both positive and negative symptoms, with oxytocin administration (Feifel et al. 2010; Rubin et al. 2010). Complementing this, our results suggest that oxytocin can have beneficial effects on emotion recognition, a well-documented deficit in this clinical population, which may have an impact on symptoms in the longer term, as differences were only evident after 3 weeks of treatment (Feifel et al. 2010), as well as contributing to better social functioning in the patients. Our study also demonstrates acute improvements in emotion recognition. This offers the potential in the future of integrating oxytocin treatment with psychological therapy using the window of opportunity created by the acute oxytocin effects.
Acknowledgements
The authors wish to thank all the volunteers who participated in this study. This work was supported by a Medical Research Council (MRC) project grant to S. S. S. and this work was also supported in part by the Intramural Program of the National Institutes of Health (NIH), National Institute of Mental Health.
Declaration of Interest
S. S. S. has received compensation from GlaxoSmithKline over the past 3 years.
References
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bediou B, Asri F, Brunelin J, Krolak-Salmon P, D'Amato T, Saoud M, Tazi I. Emotion recognition and genetic vulnerability to schizophrenia. British Journal of Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- Bediou B, Franck N, Saoud M, Baudouin JY, Tiberghien G, Daléry J, d'Amato T. Effects of emotion and identity on facial affect processing in schizophrenia. Psychiatry Research. 2005;133:149–157. doi: 10.1016/j.psychres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment. Oxford University Press; New York: 1994. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Mehta D. British Medical Association & the Royal Pharmaceutical Society of Great Britain. British National Formulary: 54. BMJ Publishing Group; London: 2007. ( ed. [Google Scholar]
- Burbach JP, Young LJ, Russell J. Neill J. D. Knobil and Neill's Physiology of Reproduction. 3rd edn. Elsevier; London: 2006. Oxytocin: synthesis, secretion and reproductive functions; pp. 3055–3128. ( ed. ), pp. [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, Hulsmann A, Schneider F, Falkenberg DI, Habel U. Generalized deficit in all core components of empathy in schizophrenia. Schizophrenia Research. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of Psychopharmacology. 2009;23:241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G Heinrichs M Gläscher J Büchel C Braus DF Herpertz SC 2007aOxytocin attenuates amygdala responses to emotional faces regardless of valence Biological Psychiatry 621187–1190. [DOI] [PubMed] [Google Scholar]
- Domes G Heinrichs M Michel A Berger C Herpertz SC 2007bOxytocin improves ‘mind-reading’ in humans Biological Psychiatry 61731–733. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clinical Psychology Review. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Constants across cultures in the face and emotion. Journal of Personality and Social Psychology. 1971;17:124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Evans S, Shergill SS, Averbeck BB. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacology. 2010;35:2502–2509. doi: 10.1038/npp.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48:179–184. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ Mitchell PB Dadds MR 2008aOxytocin increases gaze to the eye region of human faces Biological Psychiatry 633–5. [DOI] [PubMed] [Google Scholar]
- Guastella AJ Mitchell PB Mathews F 2008bOxytocin enhances the encoding of positive social memories in humans Biological Psychiatry 64256–258. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC. Facial emotion recognition in schizophrenia: intensity effects and error pattern. American Journal of Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berlin) 2010;209:225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Gaschke YN. The experience and meta-experience of mood. Journal of Personality and Social Psychology. 1988;55:102–111. doi: 10.1037//0022-3514.55.1.102. [DOI] [PubMed] [Google Scholar]
- Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Current Opinion in Psychiatry. 2009;22:140–146. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr.. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. Journal of Neuroscience. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia Research. 2010;124:13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neuroscience Letters. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Scoriels L, Barnett JH, Murray GK, Cherukuru S, Fielding M, Cheng F, Lennox BR, Sahakian BJ, Jones PB. Effects of modafinil on emotional processing in first episode psychosis. Biological Psychiatry. 2011;69:457–464. doi: 10.1016/j.biopsych.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues in Clinical Neuroscience. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiology and Behavior. 2010;101:193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neuroendocrinology. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]