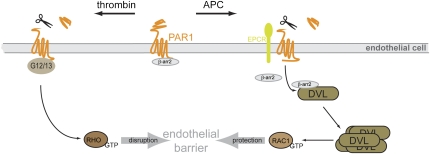
Fig. 1.
Schematic presentation of PAR1-mediated signaling pathways in endothelial cells. Thrombin binds PAR1 and activates heterotrimeric G12/13 proteins to regulate endothelial barrier disruption via the small GTPase RHOA. A second protease, APC, also acts by inducing proteolytic cleavage of PAR1 but requires the EPCR. APC induces the release of β-arrestin 2 (β-arr2) from PAR1 to associate with DVL2, which increases DVL polymerization. This is interpreted as DVL activation and is followed by GDP/GTP exchange at the small GTPase RAC1. β-Arrestin 2 and DVL2 interaction is necessary for the APC-induced endothelial barrier protection, which has therapeutic implications for the regulation of coagulation and anticoagulation. The discovery of the unexpected liaison between β-arrestin 2 and DVL downstream of PAR1 presents a unique signaling paradigm and opens the possibility for additional crosstalk between classical 7TMRs and WNT signaling.