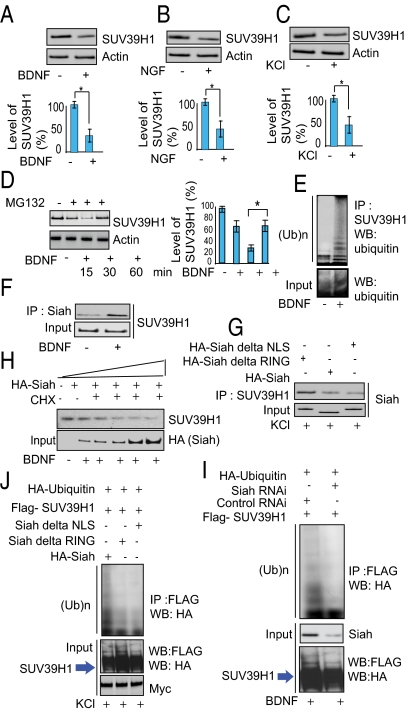
Fig. 3.
Siah is required for ubiquitination of SUV39H1. (A–C) Immunoblot analysis of SUV39H1 in BDNF- (A) and NGF- (B) treated primary cortical neuronal cells and in KCl-treated (C) PC-12 cells. Densitometric analysis of SUV39H1 in BDNF, NGF, and KCl treated cells: *P < 0.01, n = 3, one-way ANOVA, mean ± SEM. (D) Proteasome inhibition leads to SUV39H1 up-regulation. Primary neurons were treated with MG132 as indicated. Endogenous SUV39H1 and actin were detected by Western blots. *P < 0.01, n = 4, one-way ANOVA, mean ± SEM. (E) SUV39H1 is polyubiquitinated endogenously in primary neurons upon treatment with BDNF. Ten percent of the input lysate was analyzed by Western blot (WB) (Lower). IP, immunoprecipitation; (Ub)n, polyubiquitin chains. (F) Siah–SUV39H1 binding in primary neurons treated with BDNF. Cell lysates were immunoprecipitated (IP) with an anti-Siah lysine antibody, and the immunoprecipitates were analyzed by Western blotting with an anti-SUV39H1 antibody. (G) Siah lacking the NLS or RING domain does not bind SUV39H1. (H) SUV39H1 is degraded by Siah in PC-12 cells. SUV39H1 and actin proteins were detected by Western blot in cycloheximide (CHX)-treated cells with increasing concentrations of HA-Siah. (I) Depletion of Siah by RNAi leads to diminished ubiquitination of SUV39H1. (J) Siah lacking the NLS or RING domain does not elicit SUV39H1 ubiquitination. PC-12 cells were transfected as indicated, and their lysates were analyzed by immunoprecipitation and Western blotting. Ten percent of the input lysate was analyzed by Western blotting with an anti-HA and anti-FLAG antibody.