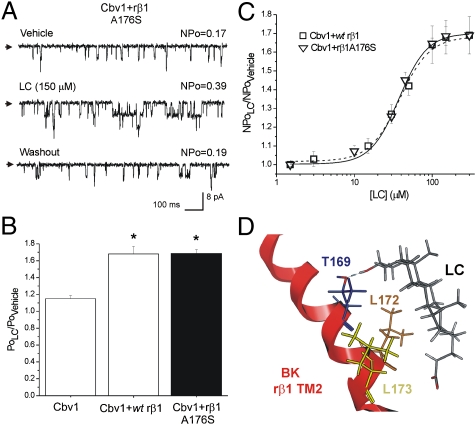
Fig. 5.
The nonconservative substitution A176S in the inner region of rβ1 TM2 domain does not ablate LC sensitivity of BK channels. Proposed model for the BK β1 TM2 domain-steroid (LC) interaction. (A) Unitary current records from cbv1 + rβ1A176S channels show increase in NPo in response to LC. (B) Averaged responses to LC. *P < 0.05; significant from control (vehicle). (C) CRCs for LC applied to cbv1 coexpressed with either WT rβ1 (dash line) or rβ1A176S show similar characteristics: EC50 = 40.4 and 37.4, respectively, Emax ∼ 300 μM, and apparent Hill number (defined as the slope of the logit-log plot of LC action on channel steady-state activity) is ∼1.32 for each construct. (D) Model of BK β1 TM2 domain steroid (LC) interaction. Hydrogen bond between TM2 Thr169 and C3-hydroxyl of LC is indicated as a blue dashed line.