Abstract
T cells and endothelin (ET-1) both contribute to angiotensin II (AngII)-dependent hypertension. To determine whether ET-1, via the ETA receptor, facilitates T cell infiltration in the kidney during AngII-dependent hypertension, we measured T cell infiltration in response to four different treatments: saline, AngII infusion, AngII infusion with an ETA receptor antagonist, or AngII infusion with triple-antihypertensive therapy. After 14 days, AngII increased both BP and the numbers of CD3+ and proliferating cells in the kidney. Mice treated concomitantly with the ETA receptor antagonist had lower BP and fewer CD3+ and proliferating cells in the renal cortex. Mice treated with triple therapy had similar reductions in BP but no change in renal cortical CD3+ cells compared with kidneys from AngII-infused hypertensive mice. In the outer medulla, both the ETA receptor antagonist and triple therapy reduced the number of CD3+ cells and macrophages. Taken together, these data suggest that ETA receptor activation in AngII-mediated hypertension increases CD3+ cells and proliferation in the renal cortex independent of changes in BP, but changes in the number of inflammatory cells in the renal medulla are BP dependent.
The kidney plays a pivotal role in the regulation of BP by controlling sodium balance via both renal hemodynamic and tubular mechanisms.1,2 Hypertension and its associated pathologies have complex etiologies, and recent evidence indicates that inflammatory-mediated processes are involved.3 An intriguing but as yet uninvestigated interaction involves T cells and endothelin (ET-1). The mechanisms influencing renal inflammation in hypertension, including a role for ET-1, have not been established.
ET-1 is a peptide produced by a variety of cell types including endothelial cells and inner medullary collecting duct cells.4 ET-1 production is triggered by multiple stimuli including angiotensin II (AngII) and proinflammatory cytokines.5,6 Two receptor subtypes, ETA and ETB, account for the wide range of effects of ET-1.7 ETA receptor blockade attenuates AngII-induced hypertension, supporting a role for ET-1 in the pathogenesis of chronic AngII-dependent hypertension.8–12 ET-1 via activation of ETA receptor, in conjunction with AngII, also stimulates cell proliferation and promotes inflammation in the kidney.13,14
Shao et al.15 showed that AngII alters the balance of T cell subsets in the kidney, providing the first evidence that T cells participate in AngII-dependent hypertension. More recently, Guzik et al.16 demonstrated a critical role of T cells in AngII-induced hypertension, reporting that AngII-induced hypertension is attenuated in mice lacking T and B lymphocytes (Rag 1−/−), and adoptive transfer of T cells, but not B cells, restored the full hypertensive effects of chronic AngII infusion. T cells in the kidney have been linked with renal injury in AngII-dependent hypertension17 and the pathogenesis of genetic salt-sensitive hypertension,18 but the factors governing immune cell infiltration of the kidney in AngII-dependent hypertension are unknown.
Despite the compelling evidence that T cells and ET-1 contribute to the mechanism of AngII-dependent hypertension, the interplay between these factors has not been investigated. Our overall hypothesis is that ETA receptor activation and not increased BP, per se, mediates inflammatory cell infiltration and/or proliferation in the kidney during AngII-induced hypertension. This hypothesis was tested by infusing mice with AngII in the presence and absence of a selective ETA receptor antagonist or triple therapy and determining the number of T cells, monocytes/macrophages, and proliferating cells in the renal cortex and medulla by immunohistological analysis.
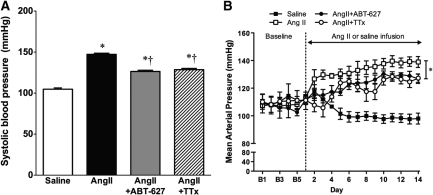
Infusion of AngII in C57Bl/6 mice for 2 weeks significantly increased BP compared with saline (Figure 1). Concurrent administration of the selective ETA antagonist ABT-627 during AngII infusion significantly attenuated the hypertensive response to AngII. This attenuation rather than complete prevention of AngII-dependent hypertension by ETA antagonism is consistent with results previously obtained by others using a different ETA antagonist in rats.9,11 Elegant studies from the Cowley laboratory utilizing a servo-controlled occluder placed around the aorta above the level of the renal arteries have demonstrated that elevated renal perfusion pressure and not a direct action of AngII accounts for a large portion of renal injury in the chronic AngII infusion model in the rat.19,20 There have been no similar studies in the mouse. Because ETA receptor blockade attenuated the hypertensive effect of AngII, an additional group of AngII-infused mice received “triple therapy” (combined reserpine, hydralazine, and hydrochlorothiazide) to reduce pressure to the same level as with ABT-627 (Figure 1).
Figure 1.
BP in saline-, angiotensin-II (AngII)-, AngII+ABT-627-, and AngII+TTx-treated mice. (A) Systolic BP at day 13 of AngII or saline infusion measured by tail cuff (n = 8 to 10). (B) Twenty-four-hour average mean arterial pressure (MAP) before (baseline) and during AngII or saline infusion measured by telemetry (n = 4 to 5). Vertical line and asterisk indicate all three AngII-infused groups at day 14 are significantly different from saline. Data are means ± SEM. *P < 0.05 versus saline; †P < 0.05 versus AngII.
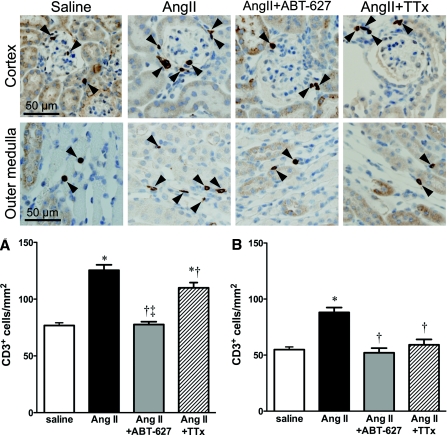
T cells were quantified by counting CD3+ cells within the renal cortex and outer medulla, separately. The number of CD3+ cells in both the cortex and medulla was significantly increased in AngII-infused mice compared with all other groups (Figure 2). ETA receptor blockade reduced the number of CD3+ cells within the kidney of AngII-infused mice. The triple therapy group displayed more CD3+ cells in the renal cortex than the ABT-627-treated group (Figure 2A). Therefore, pressure alone does not appear to account for increased T cell numbers in the renal cortex of AngII-infused mice. In contrast, the number of CD3+ cells in the outer medulla was reduced in both ABT-627 and triple therapy groups (Figure 2B), consistent with a pressure-dependent change in T cell number within the medulla. Additional characterization of T cell subsets was performed, revealing a qualitatively similar pressure-dependent pattern of CD4+ cell infiltration in both cortex and medulla (Supplemental Figure 1). CD8+ cell numbers were not significantly different among the four groups in either cortex or medulla, although cell numbers tended to be higher in the renal cortex of the saline group compared with the other three groups (Supplemental Figure 1). These data indicate that BP per se influences T cell subsets within the kidney in a differential manner. It should be noted that analysis of CD3+ cells was performed using different fixation methods, antibodies, and mice compared with those used for CD4+ and CD8+ cells, and accordingly, comparison of raw cell numbers between these two data sets is not possible. However, at face value, our data do suggest that CD4+ and CD8+ cells may be present in similar or greater numbers than CD3+ cells. It has been reported that 50% of T cells found in normal mouse kidneys are so-called CD3 intermediate cells (CD3intIL-2Rb+), which express intermediate levels of the T cell receptor-CD3 complex.21 If, under our staining conditions, such cells were not detectable as CD3+ but did stain positive for CD4 or CD8, this might contribute to the seemingly high proportion of CD4+ and CD8+ cells relative to total CD3+ cells in our studies. Another possible explanation would be the presence of CD3−CD4+ and CD3−CD8+ cells in the kidneys. Lymphoid tissue inducer cells, critical for lymph node development, may be CD3−CD4+ in mice,22 and although they have been shown to persist in adults, there is a lack of data on these cells in the kidney. Interestingly, these cells produce IL-17, which has been implicated in AngII-dependent hypertension.23,24 Natural killer cells may be CD3−CD8+ in humans and rats, but reportedly not in mice25; however, the presence of some other CD3−CD8+ cell type in the mouse kidney should not be ruled out.
Figure 2.
Representative histologic images of CD3+ staining in the renal cortex and outer medulla for saline-, AngII-, AngII+ABT-627-, and AngII+TTx-treated mice. Magnification, ×40. (A) Quantification of CD3+ cells in the renal cortex. (B) Quantification of CD3+ cells in the renal outer medulla. For all groups, CD3+ were primarily localized in proximity to glomeruli in the renal cortex and vascular structures (i.e., vasa recta) in the outer medulla. Data are means ± SEM. *P < 0.05 versus saline; †P < 0.05 versus AngII; ‡P < 0.05 versus AngII+TTx (n = 8 to 10).
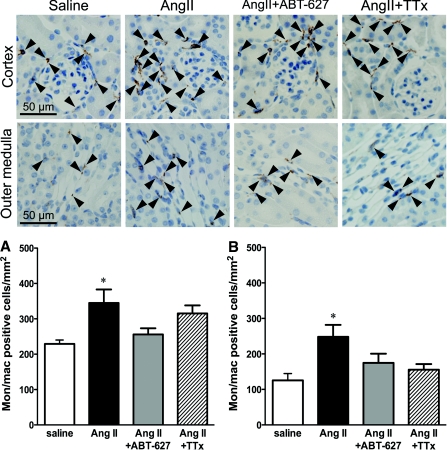
The number of monocytes/macrophages in the renal cortex and outer medulla in the AngII group was significantly increased compared with the saline group (Figure 3). In contrast to CD3+ cells, the numbers of monocytes/macrophages in ETA receptor antagonist and triple therapy groups were not significantly different from saline, suggesting that infiltration of monocytes/macrophages in both the cortex and medulla is solely BP dependent. However, although not statistically significant, triple therapy appeared less efficacious than ABT-627 in attenuating cortical monocyte/macrophage infiltration.
Figure 3.
Representative histologic images of monocyte and macrophage staining in the renal cortex and outer medulla for saline-, AngII-, AngII+ABT-627-, and AngII+TTx-treated mice. Magnification, ×40. (A) Quantification of monocyte/macrophage (mon/mac)-positive cells in the renal cortex. (B) Quantification of monocyte/macrophage (mon/mac)-positive cells in the renal outer medulla. For all groups, monocytes and macrophages were primarily localized in proximity to glomeruli in the renal cortex and vascular structures (i.e., vasa recta) in the outer medulla. Data are means ± SEM. *P < 0.05 versus saline (n = 8 to 10).
The main findings from the current study support the hypothesis that ETA receptor activation in the renal cortex during AngII hypertension increases total (CD3+) T cell numbers independent of changes in BP. Furthermore, in contrast to events that occur in the renal cortex, our data indicate that mechanisms in the outer medulla contributing to increased CD3+ cells are dependent on BP. Our data concerning CD4+ and CD8+ cells indicate that the influence of BP and indeed AngII hypertension on specific T cell subsets, however, appears to be complex, with not all subsets affected similarly. Future studies will be needed to sort out the influence of ETA receptors on T cell subsets. Macrophage infiltration in both the cortex and outer medulla is, however, mediated by the hypertension associated with AngII.
Although AngII infusion increased the number of T cells in the kidney, we cannot distinguish between T cell proliferation and increased infiltration from blood or lymphatic sources. To begin to dissect this difference, we evaluated cellular proliferation by Ki-67 immunostaining (Supplemental Figure 2). Ki-67+ immunolabeling detects a nuclear antigen in any proliferating cell and, as such, does not specifically identify proliferating cells as T lymphocytes. Increased cell proliferation within the renal cortex was found to be dependent on ETA receptor activation, similar to increased T cell numbers. Taken together, these data are consistent with ETA receptor activation stimulating T cell proliferation within the renal cortex, although the current study was not designed to allow us to draw such a conclusion definitively.
The mechanism of ETA receptor activation increasing T lymphocyte numbers and cell proliferation has not been evaluated in vivo. Several recent studies have suggested localized action of ET-1 initiates a cascade of events resulting in inflammation in the absence of elevated BP. Amiri et al.26 demonstrated local vascular proinflammatory and promitogenic effects (increased activation of nuclear transcription factors and expression of vascular adhesion molecules) of ET-1 independent of its effect on BP and systemic inflammation. Recently, our laboratory has found that ET-1 infusion for 2 weeks increases renal cortical expression of adhesion molecules and proinflammatory mediators without increasing BP.27 Very little is known about ETA receptor expression and/or activation pathways in T cell subpopulations. Our results thus far suggest that activation of CD4+ cells is pressure dependent, rather than mediated by ET-1. Future studies will begin to address ET-1-mediated T cell activation pathways, further identify the subtypes of T cell involvement, and more importantly, the functional consequences of the increased T cell numbers in the renal cortex.
Mechanisms underlying pressure-dependent inflammatory cell proliferation and/or infiltration in the kidney have yet to be elucidated. Mori et al.19 demonstrated outer medullary injury in AngII-dependent hypertension is initiated by increased perfusion pressure followed by increased levels of proinflammatory mediators. Our data indicate that the increases in CD3+ cells in the outer medulla are dependent on changes in BP and not ETA receptor activation. The differences in the pressure dependency of T cell numbers in the renal cortex versus medulla may be related to differential localization of ETA and ETB receptors in the kidney. ETA receptors constitute approximately 70% of the total ET-1 binding in the renal cortex, yet only about 30% in the renal medulla. Therefore, one would expect a greater influence of ETA receptors in the cortex compared with the medulla.
De Ciuseis et al.28 reported that AngII-induced hypertension is mediated, in part, by macrophages, because osteopetrotic (Op/Op) mice, which are deficient in macrophage colony-stimulating factor do not develop AngII-dependent hypertension. Our study uncovered an important role for BP in driving monocyte/macrophage infiltration of the kidney. Together, these data suggest that not only are macrophages important mediators of hypertension, but that their behavior is also affected by hypertension. In contrast, our data suggest that the mechanisms underlying hypertension-induced increases of T cells and macrophages in the renal cortex may be distinct. A possible explanation for this difference may be related to separate activation pathways that mediate T cell proliferation and macrophage infiltration.
In conclusion, data from this study has provided new insight into the mechanisms by which ETA receptor activation contributes to the development and/or maintenance of hypertension. The data indicate that ETA receptor activation increases CD3+ T cell numbers in the renal cortex and that this is via a BP-independent mechanism. The precise subsets of T cells affected by ETA receptor activation await future investigation. ET receptor antagonists are utilized for pulmonary hypertension, and a selective ETA receptor antagonist is currently in clinical trials with promising results, in patients with resistant hypertension.29 Our data would suggest that ETA receptor antagonists may be more efficacious in this subset of patients by diminishing the involvement of T lymphocytes in chronic renal injury. We also found that increased BP induces increased numbers of T cells in the outer medulla and monocytes/macrophages in both the renal cortex and outer medulla. These studies highlight novel therapeutic approaches for hypertension and/or renal end organ damage by targeting specific endothelin and/or T cell activation pathways.
CONCISE METHODS
Animals
All experiments were performed using 10-week-old male C57Bl/6 mice (22 to 25 g; Jackson Laboratories) in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Medical College of Georgia Institutional Animal Care and Use Committee. Mice were randomly assigned to one of four study groups: control group received saline infused via a micro-osmotic pump (Alzet model 1002) for 14 days (saline); AngII (490 ng/kg per minute; Phoenix Pharmaceuticals) infused by the same method for 14 days (AngII); AngII infusion plus treatment with either an ETA receptor antagonist (ABT-627 5 mg/kg per day; AngII+ABT-627; Abbott Laboratories), or triple antihypertensive therapy30 (hydralazine 30 mg/kg per day, reserpine 0.6 mg/kg per day, and hydrochlorothiazide 12 mg/kg per day; AngII+TTx; Sigma Chemicals). ABT-627 shows 1000-fold selectivity for the ETA receptor over the ETB receptor,31 and the dose chosen has previously been used by others in mice.32,33 Drugs were given in drinking water throughout the AngII infusion period. Mice in the treatment groups drank similar amounts of water (5 ml/d).
Blood Pressure Measurements
Systolic BP was monitored by noninvasive computerized tail-cuff method (Visitech BP-2000 Series II Blood Pressure Analysis System) throughout the infusion period. After a 2-week training period, baseline measurements were taken 1 day before osmotic mini-pump implantation and then again on either day 3 and/or 7, with the final systolic BP measured on day 13 of infusion. In a second series of experiments, mean arterial pressure was measured via telemetry as described previously.5
Immunohistochemical Analyses
On day 14 of infusion, mice were anesthetized, and kidneys were perfused with PBS and fixed with 10% buffered formalin at 110 mmHg as described previously.8,34 Briefly, kidneys were immersed in 4% buffered formalin solution overnight at room temperature, transferred to 70% ethanol for 24 hours, and paraffin-embedded. Sections were stained with primary antibodies specific for T cells (CD3, 1:2500; Santa Cruz Biotechnology) overnight at 4°C and detection with the immunocruz staining system (Santa Cruz Biotechnology) or monocytes/macrophages (F4/80, 1:100; Serotec or RM0029, 1:100; Abcam) overnight at 4°C and detection with horseradish peroxidase-conjugated secondary antibodies (Serotec and Abcam, respectively) for 1 hour at room temperature and detection with polymer conjugated secondary antibody (BioCare Medical). RM0029 is specific for rodent macrophage antigen, whereas F4/80 detects rodent monocyte/macrophage antigen. Quantitative assessment of macrophages was completed with the RM0029 staining. F4/80 specific staining gave qualitatively similar results. Methods for CD4+ and CD8+ staining and cell counting are given in the data supplement.
CD3+ cells, monocyte/macrophage positive cells, and Ki-67+ cells were counted in 10 separate 200 × 200-μm microscopic fields in four separate sections of kidney cortex and outer medulla (magnification: ×40) in a blinded manner. The final reported cell number was the average of the four separate sections in the cortex and outer medulla of each treatment group.
Statistical Analyses
All data are expressed as means ± the SEM. P < 0.05 was considered statistically significant. Comparisons between treatment groups were made by a one-way ANOVA, followed by post hoc analysis using the Tukey-Kramer test.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We acknowledge funding for this research from National Institutes of Health Grants HL74167 (DMP), HL60653 (JSP), and HL69999 (DMP/JSP) and the Medical College of Georgia Cardiovascular Discovery Institute. We thank Janet Hobbs, Hiram Ocasio, and Dr. Kelly Hyndman for the expert technical assistance and helpful discussions.
Portions of this work were previously presented at the 2009 Annual Meeting of the American Society of Nephrology (J Am Soc Nephrol 20: 558A, 2009).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Guyton AC, Coleman TG, Young DB, Lohmeier TE, DeClue JW: Salt balance and long-term blood pressure control. Annu Rev Med 31: 15–27, 1980 [DOI] [PubMed] [Google Scholar]
- 2. Hall JE, Guyton AC, Coleman TG, Mizelle HL, Woods LL: Regulation of arterial pressure: Role of pressure natriuresis and diuresis. Fed Proc 45: 2897–2903, 1986 [PubMed] [Google Scholar]
- 3. Schiffrin EL: T lymphocytes: A role in hypertension? Curr Opin Nephrol Hypertens 19: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Schneider MP, Boesen EI, Pollock DM: Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 47: 731–759, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM: Interleukin-1β but not interleukin-6 enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol 295: F446–F453, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sprague AH, Khalil RA: Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539–552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohan DE: Endothelin, hypertension and chronic kidney disease: New insights. Curr Opin Nephrol Hypertens 19: 134–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ballew JR, Fink GD: Role of ET(A) receptors in experimental ANG II-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 281: R150–R154, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Barton M, d'Uscio LV, Shaw S, Meyer P, Moreau P, Luscher TF: ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension 31[1 Pt 2]: 499–504, 1998 [DOI] [PubMed] [Google Scholar]
- 10. d'Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Luscher TF: Effects of chronic ETA-receptor blockade in angiotensin II-induced hypertension. Hypertension 29[1 Pt 2]: 435–441, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Moreau P, d'Uscio LV, Shaw S, Takase H, Barton M, Luscher TF: Angiotensin II increases tissue endothelin and induces vascular hypertrophy: reversal by ET(A)-receptor antagonist. Circulation 96: 1593–1597, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG: Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension 30[1 Pt 1]: 29–34, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Epstein BJ, Anderson S: Endothelin receptor antagonists as antihypertensives: The next frontier. Expert Rev Cardiovasc Ther 7: 675–687, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ: Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 52: 452–459, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T: Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG: Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM: Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Miguel C, Das S, Lund H, Mattson DL: T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori T, Cowley AW, Jr: Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Polichnowski AJ, Cowley AW, Jr: Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension 54: 1269–1277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, Soloski MJ, Rabb H: Normal mouse kidneys contain activated and CD3+CD4- CD8- double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Withers DR: Lymphoid tissue inducer cells. Curr Biol 21(10): R381–R382, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ: Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206: 35–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG: Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seaman WE: Natural killer cells and natural killer T cells. Arthritis Rheum 43: 1204–1217, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Amiri F, Paradis P, Reudelhuber TL, Schiffrin EL: Vascular inflammation in absence of blood pressure elevation in transgenic murine model overexpressing endothelin-1 in endothelial cells. J Hypertens 6: 1102–1109, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM: Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56: 942–949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL: Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: Evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25: 2106–2113, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Prasad VS, Palaniswamy C, Frishman WH: Endothelin as a clinical target in the treatment of systemic hypertension. Cardiol Rev 17: 181–191, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H: Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol 36: 750–755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winn M, von Geldern TW, Opgenorth TJ, Jae HS, Tasker AS, Boyd SA, Kester JA, Mantei RA, Bal R, Sorensen BK, Wu-Wong JR, Chiou WJ, Dixon DB, Novosad EI, Hernandez L, Marsh KC: 2,4-Diarylpyrrolidine-3-carboxylic acids—potent ETA selective endothelin receptor antagonists. 1. Discovery of A-127722. J Med Chem 39: 1039–1048, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Giachini FR, Zemse SM, Carneiro FS, Lima VV, Carneiro ZN, Callera GE, Ergul A, Webb RC, Tostes RC: Interleukin-10 attenuates vascular responses to endothelin-1 via effects on ERK1/2-dependent pathway. Am J Physiol Heart Circ Physiol 296: H489–H496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amiri F, Ko EA, Javeshghani D, Reudelhuber TL, Schiffrin EL: Deleterious combined effects of salt-loading and endothelial cell restricted endothelin-1 overexpression on blood pressure and vascular function in mice. J Hypertens 28: 1243–1251, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS: Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.