Figure 3.
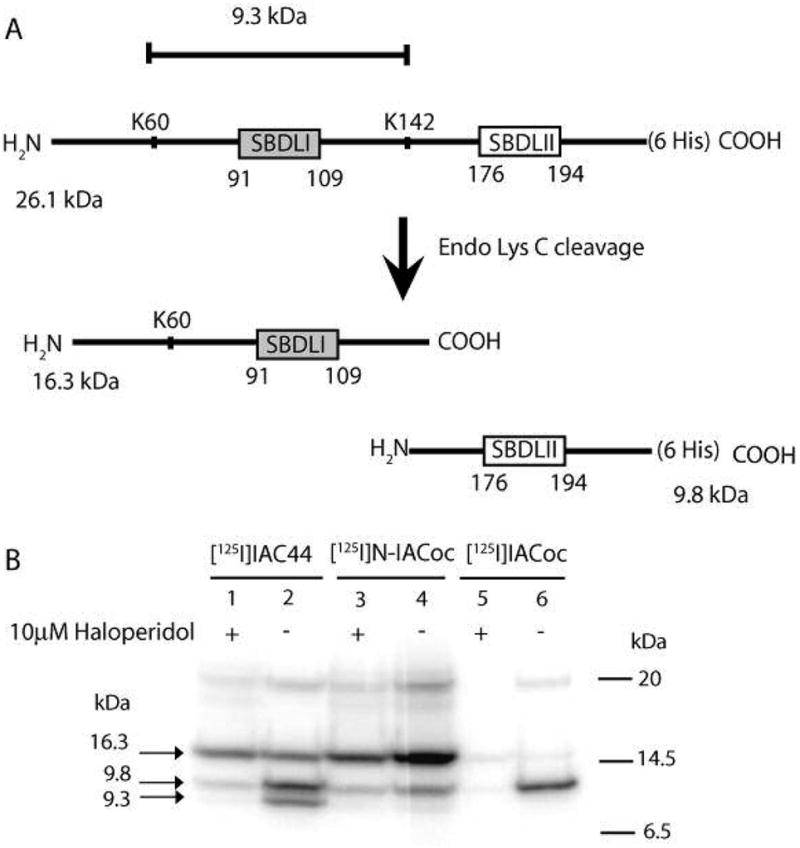
(A) Schematic diagram of Endo Lys C cleavage of the pure guinea pig sigma-1 receptor. (B) Autoradiogram of Endo Lys C cleaved [125I]IAC44 (lanes 1 and 2), [125I]-N-IACoc (lanes 3 and 4), and [125I]IACoc (lanes 5 and 6) photolabeled peptides. Specific labeling was determined by comparing sigma-1 receptor photolabeling in the absence (lanes 2, 4, and 6) and presence of 10 μM haloperidol (lanes 1, 3, and 5). Upon Endo Lys C cleavage, the [125I]IAC44 (lanes 1 and 2), [125I]-N-IACoc (lanes 3 and 4), and [125I]IACoc (lanes 5 and 6) photolabeled receptors resulted in two peptides with molecular masses of 16.3 and 9.8 kDa. The [125I]IAC44 (lanes 1 and 2) photolabeled receptor was also cleaved by Endo Lys C to produce a 9.3 kDa peptide. As expected, the specific radiolabel from [125I]IACoc was located on the SBDLII region (9.8 kDa) (lanes 5 and 6). The specific [125I]-N-IACoc labeled sigma-1 receptor (lanes 3 and 4) afforded radiolabel on the SBDLI (16.3 kDa) containing peptide. Alternatively, the specific [125I]I-AC44 labeled sigma-1 receptor (lanes 1 and 2) showed radiolabel on the SBDLI (16.3 kDa) containing peptide and the SBDLII (9.8 kDa) containing peptide. An additional specific radiolabeled band at 9.3 kDa (lane 2) was observed with [125I]IAF, consistent with the cleavage at position 60 by Endo Lys C, which contains the SBDLI domain.
