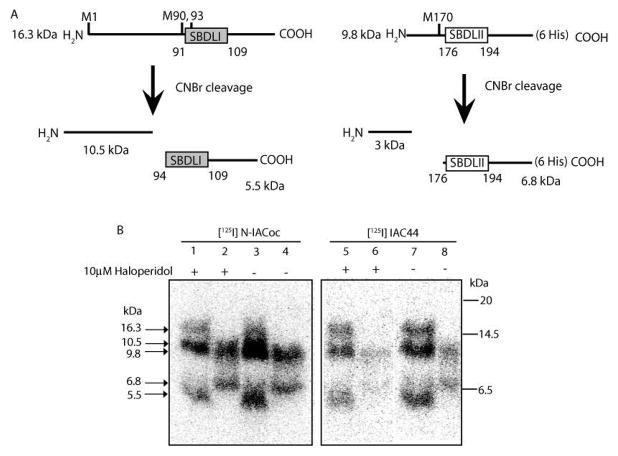
Figure 4.
(A) Schematic diagram depicting CNBr cleavage of the 16.3 and 9.8 kDa peptides derived from Endo Lys C cleavage of pure guinea pig sigma-1 receptors. (B) Autoradiogram of CNBr-cleaved [125I]IAC44 (lanes 1–4) and [125I]-N-IACoc (lanes 5–8) photolabeled peptides from Endo Lys C cleavage (16.3 and 9.8 kDa). Specific labeling was determined by comparing sigma-1 receptor photolabeling in the absence (lanes 3, 4, 7, and 8) and presence of 10 μM haloperidol (lanes 1, 2, 5, and 6). Upon treatment with CNBr, the 16.3 kDa peptides resulted in two peptides with molecular masses of 10.5 and 5.5 kDa (lanes 1, 3, 5, and 7). Likewise, the 9.8 kDa Endo Lys C peptide resulted in a 6.8 kDa peptide after CNBr cleavage (lanes 2, 4, 6, and 8). When [125I]-N-IACoc was used as the photoprobe, the majority of haloperidol-protectable label was found on the 10.5 kDa fragment, with some protectable labeling of the 5.5 kDa peptide (lanes 1 and 3). Photolabeling of the 9.8 and 6.8 kDa bands (lanes 2 and 4) was not specific. When [125I]IAC44 was used as the photoprobe, haloperidol-protectable label was found on the 10.5, 5.5, and 6.8 kDa fragments (lanes 5–8).