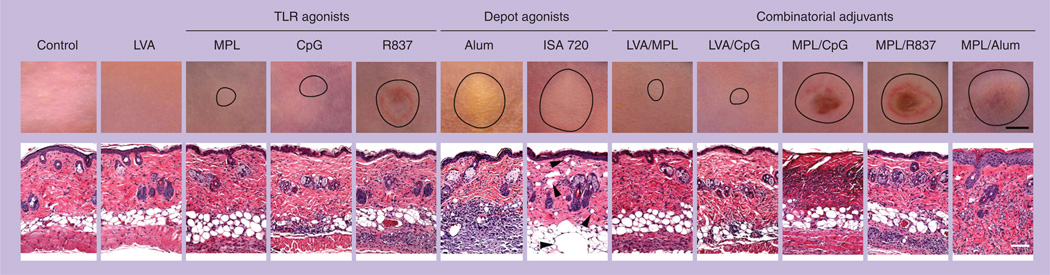
Figure 1. Reactogenicity of various vaccine adjuvants in the skin.
BALB/c mice were illuminated for 2 min with a Q-switched 532-nm Nd:YAG laser of a pulse width 5–7 ns, beam diameter 7 mm, and frequency 10 Hz at 0.3 W and 90 J/cm2 (laser vaccine adjuvant). Or, the mice were intradermally injected with indicated adjuvants with or without laser illumination. The injection volume is 20 µl containing 25 µg monophosphoryl lipid A, 30 µg CpG, 100 µg R837, 50% Alum (v/v), 70% montanide ISA 720 (v/v), or a combination of two indicated adjuvants. After 5 days, photos were taken (upper panel; scale bar: 2 mm), followed by histological examination (lower panel; scale bar: 100 µm). The lesion in the skin is outlined by circle in the upper panel and arrowheads in the lower panel of Montanide ISA 720-injected skin point to void bulbs preoccupied by the water-in-oil adjuvant.