Table 1.
Screening of Reaction Conditions for C–O Cyclization
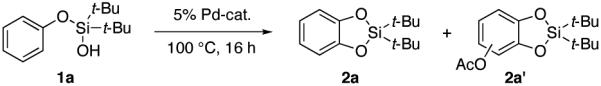 | |||||
|---|---|---|---|---|---|
| entry | Pd-cat. | oxidant | solvent | yield, %a |
|
| 2a | 2a’ | ||||
| 1b | Pd(OPiv)2 | PhI(OAc)2 (1.5 eq) | PhMe | 43 (74) | 2 |
| 2 | Pd(OPiv)2 | PhI(OAc)2 (1.5 eq) | PhMe | 50 (65) | 3 |
| 3 | Pd(OAc)2 | PhI(OAc)2 (1.5 eq) | PhMe | 40 (67) | 3 |
| 4 | Pd(OTf)2 | PhI(OAc)2 (1.5 eq) | PhMe | 40 (78) | 2 |
| 5 | Pd(OPiv)2 | PhI(OAc)2 (1.5 eq) | C6F6 | 37 (45) | 3 |
| 6 | Pd(OPiv)2 | PhI(OAc)2 (1.5 eq) | PhCF3 | 47 (53) | 14 |
| 7 | Pd(OPiv)2 | PhI(OAc)2 (2.0 eq) | PhMe | 58 (79) | 6 |
| 8 | Pd(OPiv)2 | PhI(OAc)2 (3.0 eq) | PhMe | 47 (52) | 6 |
| 9 | none | PhI(OAc)2 (1.5 eq) | PhMe | 0 | 0 |
GC yields against tetradecane as internal standard, brsm yields in the parentheses (based on recovered starting material).
Li2CO3 (1 equiv) was added.
