Table 2.
Scope of Catechol Synthesis
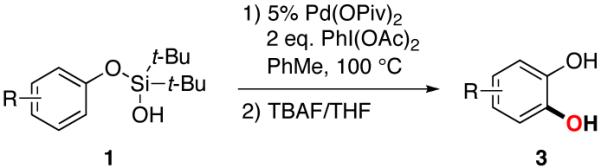 | ||||
|---|---|---|---|---|
| entry | silanol | catechol | yield, %a | |
| 1 |
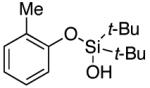
|
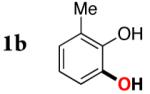
|
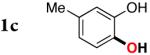
3b | 81 |
| 2 |
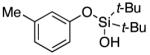
|
|
3c | 94 |
| 3 |
|
|
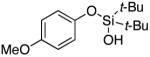
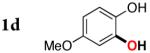
3d | 57b |
| 4 |
|
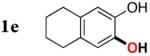
|
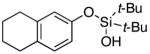
3e | 77 |
| 5 |
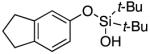
|
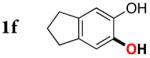
|
3f | 68c |
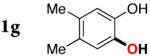
| 6 |
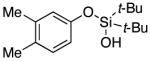
|
|
3g | 93 |
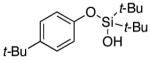
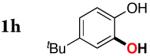
| 7 |
|
|
3h | 78 |
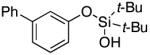
| 8 |
|
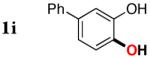
|
3i | 87 |
| 9 |
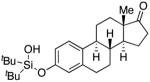
|
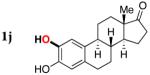
|
3j | 88 |
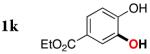
| 10 |
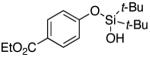
|
|
3k | 76c |
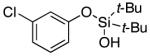
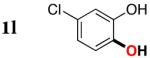
| 1l |
|
|
3l | 62b,d |
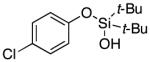
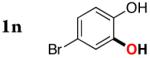
| 12 |
|
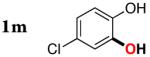
|
3m | 84b,d |
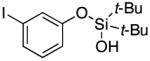
| 13 |
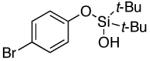
|
|
3n | 83b,d |
| 14 |
|
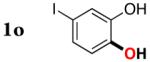
|
3o | 70b,d |
| 15 |
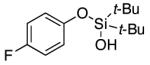
|
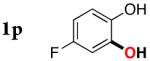
|
3p | 60b,d |
| 16 |
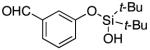
|
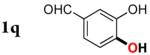
|
3q | 47b,d,e |
| 17 |
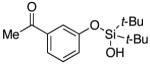
|
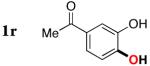
|
3r | 65b,d |
| 18 |
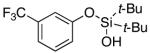
|
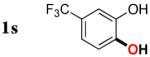
|
3s | 35b,d |
| 19 |
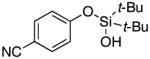
|
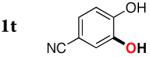
|
3t | 29b,d |
| 20 |
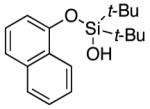
|
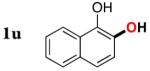
|
3u | 76b,d |
| 21 |
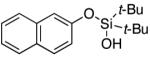
|
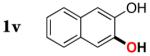
|
3v | 54b,d |
Isolated yields.
Isolated as bis-acetates by further treatment of the catechols with Ac2O and pyridine in the same pot.12
Major isomer is shown (34:1).
PhCF3 was used instead of PhMe, PhI(OAc)2 (1.5 equiv), 120 °C.
10 mol% Pd(OPiv)2 was used.
