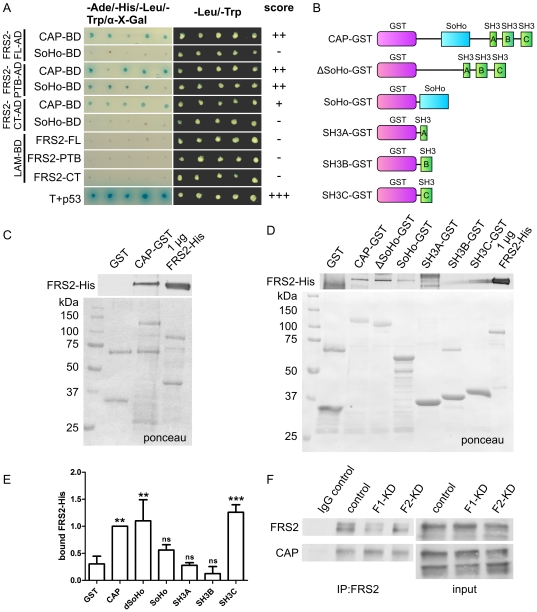
Figure 6. FRS2 directly interacts with Cbl-associated protein.
(A) Yeast two-hybrid analysis of the interaction between FRS2 and CAP domains. (B) Structure of the CAP-GST constructs used. (C) and (D) Interaction of purified FRS2-His and CAP-GST proteins. CAP-GST fusion proteins were immobilized on sepharose and tested for the binding of purified FRS2-His. Upper blot shows the bound FRS2-His (anti-His antibody), lower blot the ponceau staining of the GST proteins. 1 µg of FRS2-His was used as a positive control. (E) Quantification of the binding of FRS2 to various CAP domains. A binding of FRS2 significantly higher than background was seen with the full-length CAP, delta-SoHo and the third SH3 domain. (F) Endogenous FRS2 was immunoprecipitated from Hep3B cells, and the binding of endogenous CAP was tested. Please note that several isoforms of CAP are present in Hep3B cells, of which only one appears to bind FRS2.