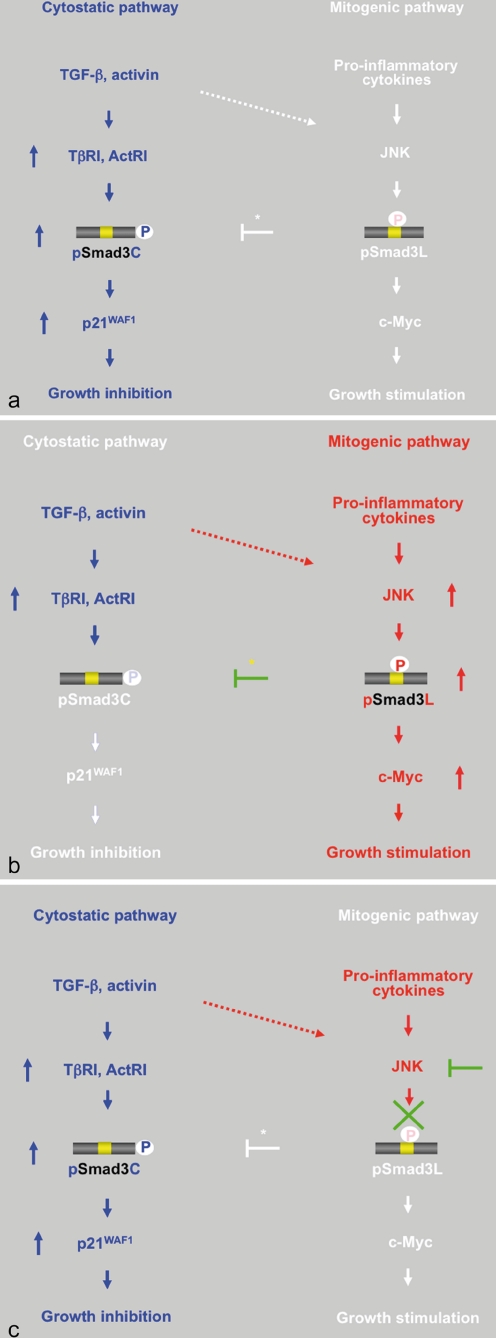
Fig. 3.
Reversible shifting of Smad3-dependent signaling between hepatocytic growth and inhibition indicates that pSmad3C transmits a cytostatic TGF-β/activin signal, whereas pro-inflammatory cytokines transmit a mitogenic signal through the JNK-dependent pSmad3L pathway. a TGF-β or activin treatment activates TβRI or ActRI, further leading to the direct phosphorylation of Smad3C. pSmad3C inhibits hepatocyte growth by up-regulating p21WAF1 transcription. b Although TGF-β and the activin signal weakly phosphorylate Smad3L in normal hepatocytes (dotted arrow), pro-inflammatory cytokines can transmit a mitogenic signal through the JNK-dependent pSmad3L pathway to participate in hepatocytic growth, possibly by stimulating the transcription of the c-Myc gene. Linker phosphorylation of Smad3 indirectly prevents its COOH-tail phosphorylation (yellow star), pSmad3C-mediated p21WAF1 transcription, and consequently the cytostatic effect of TGF-β/activin upon normal hepatocytes. c Either various JNK inhibitors or a Smad3 mutant lacking the JNK phosphorylation sites in the linker region can eliminate mitogenic pSmad3L signaling and restore the lost cytostatic pSmad3C signaling observed in mature hepatocytes