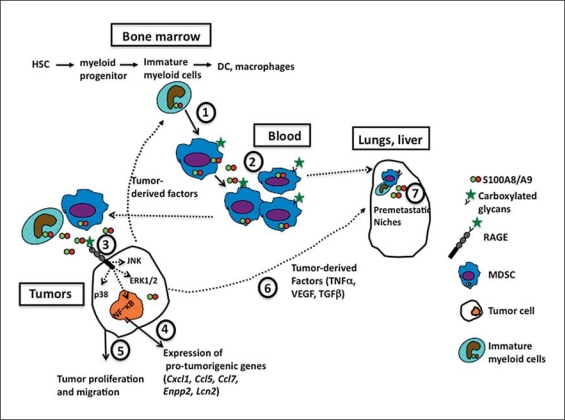
Fig. 1.
S100A8/A9 in tumor progression. (1) Tumor-derived factors promote sustained up-regulation of S100A9 in myeloid precursors in the bone marrow, which in turn inhibits normal differentiation of the precursors to dendritic cells and macrophages, and promotes generation of MDSC. (2) MDSC synthesize and secrete S100A8/A9. They also express carboxylated glycans, which provide binding sites for S100A8/A9, promoting activation of intracellular signaling pathways and supporting an autocrine feedback that causes further accumulation of MDSC. (3) MDSC migrate to tumors. At low concentrations, S100A8/A9 secreted by myeloid cells within the tumor microenvironment and by tumor cells themselves bind to RAGE on tumor cells in a carboxylated-glycan-dependent manner, promoting activation of MAPK signaling pathways and NF-κB. (4) Intracellular activation of signaling pathways enhances expression of pro-tumorigenic genes and (5) promotes tumor proliferation and migration. (6) Tumor-derived factors induce expression of S100A8/A9 in distal metastatic organs. (7) They promote formation of premetastatic niches comprising immature myeloid cells, MDSC and endothelial cells, a process dependent on S100A8/A9, SAA3 and TLR4. HSC = Hematopoietic stem cells.