Fig. 1.
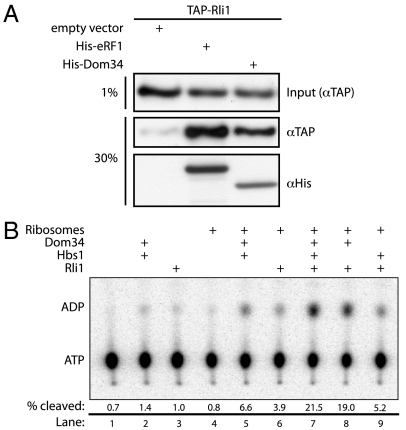
Dom34 and Rli1 interact in vivo and in vitro. (A) Interaction of Dom34 and Rli1 in extract. TAP-tagged Rli1 selectively associates with His6-tagged eRF1 or Dom34 in an Ni-NTA resin pull-down from yeast lysate. Input lysates were derived from an Rli1-TAP strain expressing either eRF1-His6, Dom34-His6, or an empty vector (pYES2) control. Both input and pelleted protein were analyzed via Western blot analysis using anti-TAP and anti-His6 antibodies. (B) Rli1 interacts productively with Dom34 and ribosomes. Multiple turnover ATPase activity of Rli1 was monitored via thin-layer chromatographic analysis of 32P-ATP hydrolysis in the presence of various factors. The fraction of ATP hydrolyzed after 15 min was quantitated and is indicated under each representative lane.