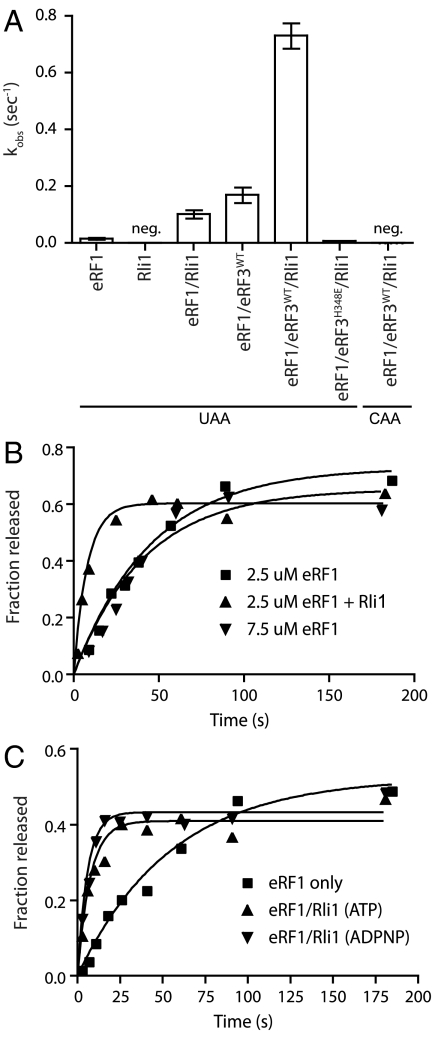
Fig. 6.
Rli1 accelerates eRF1-mediated release. (A) Observed rate constants for peptide release in the presence of eRF1, eRF3, and/or Rli1. Rates were determined using an electrophoretic TLC-based system to monitor peptide formation (n = 3, ± SEM; neg, negligible/nonmeasurable). (B) The observed effect of Rli1 on the rate of release is maintained in the presence of saturating levels of eRF1. (C) Rli1 accelerates peptide release in a step that is chemically distinct from its role in recycling. Time courses represent dipeptide formation as monitored by electrophoretic thin-layer chromatography. Both ATP and its nonhydrolyzable analog, ADPNP, allowed for Rli1-mediated stimulation of peptide release.