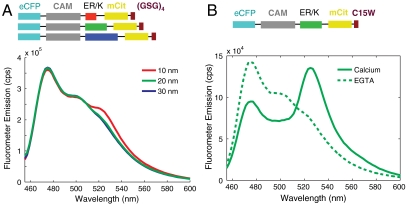
Fig. 3.
FRET as a readout of protein interaction. (A) Emission spectra of control SPASM sensors with CAM linked to a 12 amino acid Gly-Ser-Gly peptide (GSG)4 by 10-, 20-, and 30-nm ER/K α-helices. Spectra are taken at 4 mM Ca2+. Excitation of eCFP (430 nm) results in eCFP emission (475 nm) without significant mCit emission (525 nm). (B) Sample emission spectrum of SPASM sensor with CAM linked by a 20-nm ER/K α-helix to a C15W peptide (21) that interacts with 1 μM affinity to Ca2+-activated CAM. Excitation of eCFP (430 nm) results in eCFP emission (475 nm) with no detectable FRET for mCit in the absence of Ca2+ (chelation by 1 mM EGTA) or strong FRET (large mCit peak at 525 nm) in the presence of 4 mM Ca2+.