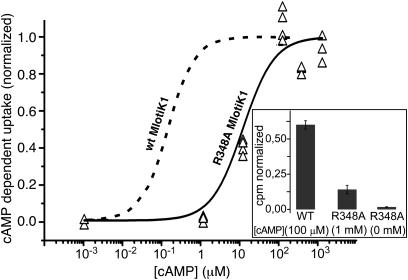
Fig. 2.
cAMP-dependent activity of the wt and R348A mutant MlotiK1 channels. Uptake of 86Rb+ into liposomes containing R348A mutant channel was measured in dependence of cAMP. The uptake activity was examined in duplicate at each cAMP concentration, from a common stock of liposomes. Raw values were first normalized by valinomycin uptake in the same vesicles and subsequently normalized by uptake at the maximum value (1 mM cAMP). The experiment was conducted twice (with different purifications), yielding four data points at each concentration. The solid curve shows a fit of the R348A data (triangles) with a Hill equation, yielding a K1/2 of activation of 10 ± 4 μM and Hill coefficient of 1.1 ± 0.6. The dashed curve illustrates the fitted curve of similar experiments published on wt MlotiK1 (5), with K1/2 of 110 nM and Hill coefficient of 1.3. The inset shows normalized radioactivity cpm for wt and mutant MlotiK1 at saturating cAMP concentrations (100 μM for wt; 1 mM for R348A) or in the absence of cAMP (R348A, 0 mM). The raw data (R348A) show mean values, and bars indicate SEs. The wt data were taken from ref. 4.