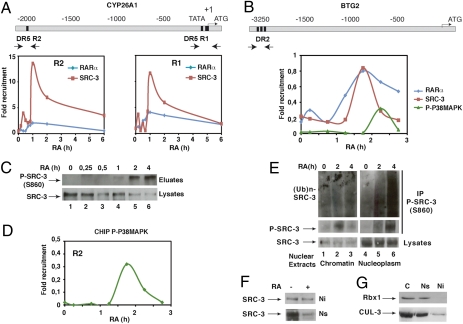
Fig. 6.
RA-induced SRC-3 ubiquitination and degradation occur out of chromatin. (A) Kinetic ChIP experiments performed with RA-treated MCF7 cells and showing the recruitment of SRC-3 and RARα to the R1 and R2 regions of the Cyp26A1 gene promoter. Values are expressed as fold enrichment relative to untreated cells and are the mean of three distinct experiments. (B) Recruitment of RARα, SRC-3, and active p38MAPK to the Btg2 gene promoter. (C) Kinetics of SRC-3 phosphorylation at S860 after phosphoprotein affinity purification and immunoblotting with the phospho-antibodies. (D) Recruitment of active phosphor-p38MAPK to the R2 region of the Cyp26A1 promoter. (E) Soluble nucleoplasmic (Ns) and insoluble chromatin (Ni) extracts were immunoprecipitated with the phospho-SRC-3 antibodies and immunoblotted with SRC-3 or ubiquitin antibodies. (F) Ni and Ns extracts were analyzed for SRC-3 degradation by immunoblotting. (G) Cytoplasmic (C), Ns, and Ni extracts were compared for the presence of RBX1 and CUL-3 by immunoblotting.