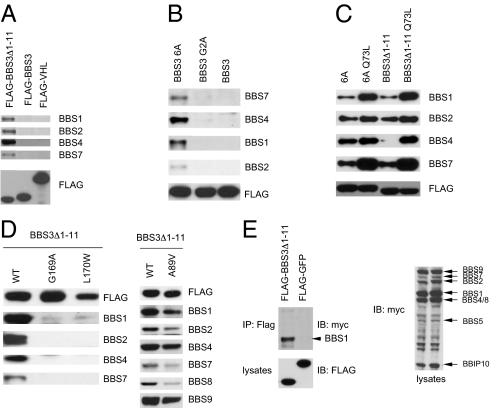
Fig. 3.
BBS3 N-terminal hydrophobic amino acids regulate BBS3–BBSome interaction, but human homozygous point mutations disrupt this interaction. (A) The N-terminal hydrophobic amino acids regulated the interaction between BBS3 and the endogenous BBSome. Shown are Western blots of Flag pull-down of 293T cells transfected with Flag-tagged full-length BBS3, BBS3 without the first 11 amino acids, and VHL as a negative control. (B) Mutation of the hydrophobic amino acids to Alanine in the first 11 amino acids of the N terminus of BBS3 increased the interaction between BBS3 and endogenous BBSome. (C) The GTP-bound form of BBS3 regulates the interaction between BBS3 and the BBSome. (D) Mutated BBS3 with mutations found in BBS patients lose the ability to interact with the BBSome but a mutation found in nonsyndromic retinal pigmentosa patients (A89V) only partially decreases the interaction between BBS3 and the BBSome. (E) BBS3 interacts with the BBSome through the BBS1 subunit. Shown are coimmunoprecipitation assays of 293T cells transfected with Flag-tagged BBS3Δ1–11 and myc-tagged BBSome subunits.