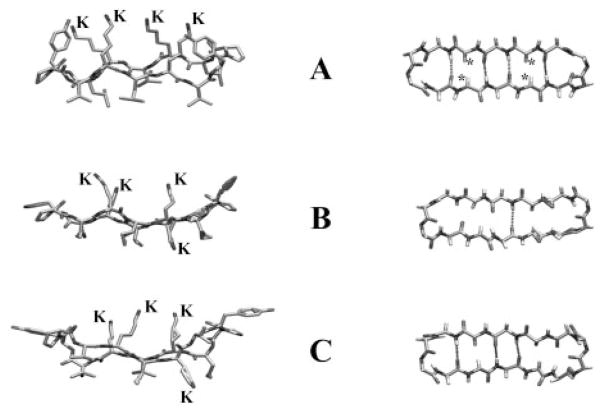
Fig. 8. Molecular models of the antibiotic peptides GS14 (A) and GS14-dK4 (B and C).
The left images show side views of the peptide backbone and the orientations of the Lys (K) and hydrophobic (unlabeled) side chains relative to the ring plane. The right images show top views of the peptide backbone with the * symbols marking the positions of the inward oriented Lys Cα protons of GS14 and the dashed lines representing connectivities where the distance and orientation of the amide carbonyls and amide protons are favorable for cross-ring hydrogen bonding. The GS14 model shown was obtained from minimized structures published by Gibbs et al. (27), and the GS14-dK4 models were obtained from the NMR solution structures in water (B) and 30% TFE (C) determined by McInnes et al. (21).