Abstract
Purpose
The lysyl oxidase (LOX) family plays a crucial role in the formation and stabilisation of extracellular matrix (ECM) by catalysing the cross-linking of collagen and elastin, implicating its important fundamental roles in injury healing. A high level of transforming growth factor-β1 (TGF-β1) accompanies the inflammatory phase of an injury of the knee joint. Our purpose was to detect the expressions of the LOX family in anterior cruciate ligament (ACL) and medial collateral ligament (MCL) response to TGF-β1.
Methods
This study used reversed transcript PCR, real time quantitative PCR and Western blot for analyses.
Results
The results showed significant increases in mRNA levels of LOX family members. At 5 ng/ml concentration of TGF-β1, the gene profiles of LOXs showed most active, and LOX and LOXL-3 showed increasing peaks at 12 hours after TGF-β1 treatment (LOX: 7.2, 8.8-fold and LOXL-3: 3.8, 5.4-fold compared with normal controls in ACL and MCL, respectively); LOXL-1, LOXL-2 and LOXL-4 reached their highest amounts at six hours (LOXL-1: 1.9, 2.4-fold; LOXL-2: 14.8, 16.2-fold; LOXL-4: 2.5, 4.4-fold in ACL and MCL, respectively). Protein assays revealed that LOXs in ACL cells had relatively lower response to TGF-β1 compared with those in MCL cells.
Conclusions
The differential expression and activities of LOXs might help to explain the intrinsic difference between ACL and MCL, and LOXs could imply a potential capability for ACL healing.
Introduction
The anterior cruciate ligament (ACL) and medial collateral ligament (MCL) are two commonly injured areas of human knee joints [1, 2]. The MCL has the capacity to self-heal and restore joint function of a completely ruptured midsubstance tear within one month, and in most cases, without the need for surgery [3]. On the other hand, the ACL does not heal satisfactorily, even if surgical repair is attempted [4].
An inflammatory response commonly occurs in the earliest phase of wound healing followed by new connective tissue matrix deposition [5]. Increases in TGF-β1 accompany the inflammatory response and appear to act as signals modulating the production of matrix macromolecules by fibrogenic cells at the site of injury [6]. Recent studies have illustrated that exogenous TGF-β1 had a strong effect on the collagen production of ACL cells [7]. Meanwhile, TGF-β1 expression could be regulated by transferring the TGF-β1 gene into ACL cells to enhance the collagen type synthesis in vitro [8]. Furthermore, TGF-β1 could promote fibronectin synthesis, which plays an important role in the complicated interaction between ligament cells and their matrix environment [9].
The lysyl oxidase family contains four additional members characterised by conserved C-terminal copper binding and functional catalytic domains. They include lysyl oxidase-like proteins 1 (LOXL-1) [10], 2 (LOXL-2) [11], 3 (LOXL-3) [12], and 4 (LOXL-4) [13], whose residue lengths are 574, 638, 753, and 756 kDa, respectively; by comparison, human prepro-LOX is 417 residues in length. Although there is little homology in the N-terminal regions of these proteins with the LOX propeptide domain, the significant homology within the C-terminal domains appears among the human LOX family [14]. Csiszar et al. [15] reported that consensus sites for cleavage by procollagen C-proteinases are absent in LOXL-2, 3, and 4, but are present in all pro-LOX enzymes and in LOXL-1; the sequences flanking the lysine and tyrosine progenitors of the LTQ cofactor in LOXL are highly conserved with those in LOX. The corresponding sequences in LOXL-2, 3, and 4 also exhibit similarities with those of LOX and LOXL-1, but are not as highly conserved.
The lysyl oxidase family can oxidise peptidyl lysine to peptidyl aldehyde residues within collagen, thus initiating formation of the covalent cross-linkages that precipitate these extracellular proteins, and fulfill the formation and repair of ECM [16]. Some reports have shown TGF-β1 has the capacity to mediate up-regulation of lysyl oxidase in the kidneys of hereditary nephrotic mouse [17] and human lung fibroblasts [18]. In many pathological fibrotic situations, the expression of LOX and its enzymatic activity are controlled by transforming growth factor (TGF)-β1 [19]. But the mechanism is still unclear on how TGF-β1 regulates LOX family expressions.
Our hypothesis is that TGF-β1 could also mediate expressions of LOXs in ACL and MCL fibroblasts, implying the important role in stabilisation of ECM and ligaments repair. In order to determine different expressions of LOX response to TGF-β1 in both ACL and MCL, we detected all members of the lysyl oxidase family using reversed transcript PCR and real time quantitative PCR and Western blot. These findings of different increases of LOXs in ACL and MCL contribute to intrinsic differences and different healing abilities between ACL and MCL.
Materials and methods
Cell culture
Human ACL and MCL cells were harvested from four donor tissues (age range 30–60, two male and two female). The ligament tissue was isolated within 12 hours of surgical intervention and immediately washed with l × PBS plus penicillin and streptomycin (200U/ml) and then cut into small pieces of 2 × 2 × 2 mm. The small pieces of ligament tissue were suspended in 10% FBS-DMEM and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After the ligament cells migrated out of the tissues and attached to the bottom, the tissues were transferred to another flask, letting the remaining cells grow to confluence (85–95%). The cells were then frozen into arrest with liquid nitrogen until use. Cells were cultured in 10% FBS media (low glucose DMEM, 0.1 mM nonessential amino acids, 4 mom L-glutamine, and antibiotics) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Only cells from passage 1 to passage 5 were used in the experiments.
TGF-β1 treatment
Cells were trypsinised and seeded on six-well plates at the concentration of 5 × 105 cells per plate (85–95% confluence). Cells were allowed 16 hours to seed and equilibrate. The culture media was removed and replaced by 2% FBS medium for 16 hours for starvation. Immediately before being treated with TGF-β1 at different concentrations (1 ng/ml, 5 ng/ml, 50 ng/ml), the culture media was replaced with fresh 1% FBS media. Samples of 600-μL culture media were collected at zero hours and 24 hours for Western blot; 800-μL cell lysate samples (according to the RNA Bio-teke Isolation Kit) were collected at zero, two, six, 12 and 24 h at 5 ng/ml TGF-β1; then 800-cell lysate samples were collected at two hours after treatment with 1 ng/ml, 5 ng/ml and 50 ng/ml TGF-β1.
RNA isolations and first-strand cDNA synthesis
Total RNA was isolated from ACL and MCL fibroblasts using Rneasy Plus Mini Kit (Qiagen, German) according to manufacturer’s instructions. RNA samples were quantified using the Nano Drop® spectrophotometer and stored at −80°C DNase I (MBI, UK); digestion was performed before the cDNA synthesis. Then, 20 μl cDNA was synthesised from 1 μg RNA using the reverse transcription kit (MBI), according to the manufacturer’s instructions.
Reversed-transcript PCR
To evaluate the expression levels of TGF-β1-induced LOX family members in both ACL and MCL normalized to the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (or beta-actin gene), RT-PCR was performed with a PCR kit (MBI) using thermo-cycler (Bio-Rad) according to described techniques. PCR primer pairs of these genes were designed using Primer5.0. The selected sets of primers are shown in Table 1. The BLAST was used to search for all the primer sequences to ensure gene specificity. RT-PCR reactions were performed in a 25-μl volume containing a 1 μl cDNA sample following the manufacturer’s recommendations. PCR consisted of an initial 30 seconds denaturising at 94°C, 30 seconds for annealing at 55–64°C and 72°C, 30 seconds for elongation, and 25–32 amplification cycles. Products were resolved by 2% agarose gel electrophoresis in Tris-borate/EDTA buffer and visualised by staining with ethidium bromide.
Table 1.
Primers design for RT-PCR and real-time quantitative PCR
| Protein | Primer pairs |
|---|---|
| GAPDH | Forward: 5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Reverse: 5′-TGGTGAAGACGCCAGTGGA-3′ | |
| LOX | Forward: 5′-GCATACAGGGCAGATGTCAGA-3′ |
| Reverse: 5′-TTGGCATCAAGCAGGTCATAG-3′ | |
| LOXL-1 | Forward: 5′-TGCCACCAGCATTACCACAG-3′ |
| Reverse: 5′-GAGGTTGCCGAAGTCACAGG-3′ | |
| LOXL-2 | Forward: 5′-CTGCAAGTTCAATGCCGAGT-3′ |
| Reverse: 5′-TCTCCACCAGCACCTCCACTC-3′ | |
| LOXL-3 | Forward: 5′-CAACAGGAGGTTTGAACGCTAC-3′ |
| Reverse: 5′-GCTGACATGGGTTTCTTGGTAA-3′ | |
| LOXL-4 | Forward: 5′-TTCACCCACTACGACCTCCTCA-3′ |
| Reverse: 5′-CAGCAGCCTACAGTCACTCCCT-3′ |
Real-time quantitative PCR
Real-time quantitative PCR was performed with QuantiTect SYBR Green PCR kit (Qiagen, German) using iCycler (Bio-Rad) according to described techniques. RT-PCR reactions were performed at 0.5 μM for each primer in a 25 μl volume containing a 1 μl cDNA sample. The reaction was initiated by activating the polymerase with a five minute pre-incubation at 95°C. Amplification was achieved with 42 cycles of 15 seconds denaturation at 94°C, 20–30 seconds annealing at 64°C and 10 seconds extension at 72°C. The program was concluded by a melting curve analysis. All experiments were performed in triplicate. The copy numbers of each gene were determined with cycle threshold (CT) methods. The means of the copy numbers of GAPDH were used as internal controls to normalise the data. Standards for establishing standard curves of all primers were prepared from total normal RNA, amplified by RT-PCR, and cloned using TOPO II TA cloning kit (Invitrogen) following the manufacturer’s recommendations.
Western blotting
Protein samples were prepared by mixing one part of sample with one part of Bio-Rad Laemmli sample buffer and then boiled at 100°C for three minutes. Proteins were separated in 10% SDS-PAGE and transferred to a PVDF membrane at 200 mA for two hours at room temperature (RT). The blot was blocked with 5% non-fat dry milk suspended in 1 × TBST for two hours at room temperature. The resulting blot was incubated with 1:1000 goat LOX polyclonal antibody from Santa Cruz Biotechnology (H-76, sc-10736) for two hours at RT, followed by incubation with 1:5000 rabbit anti-goat IgG-HRP from Santa Cruz Biotechnology for two hours at RT. Between the first and second incubation, the blot was washed three times with 1 × TBST for five minutes each time. Signals from blots were obtained using Santa Cruz Western Blotting Luminol Reagent Kit (sc-2048). Proteins were visualised by chemiluminescence with hydrogen peroxide using Kodak X-AR and luminol as substrate.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) to determine whether differences existed among groups. Post-hoc analysis used Fisher’s protected least significant differences (PLSD). In each analysis, critical significant level was set at α = 0.05.
Results
Cell viability
No cytotoxic effects of exogenous growth factor TGF-β1 were observed on the ACL and MCL cells at the doses used in this study (data not shown). Additionally, increasing dose of TGF-β1 did not significantly alter cell viability in our lab.
The LOX family members were all expressed in normal ACL and MCL fibroblasts
In cultured cells, we found all family members were expressed in both normal ACL and MCL through reversed transcript PCR, real time quantitative PCR and Western blot. The real time PCR results showed all family members are expressed higher in MCL than those in ACL, 2 ± 0.3-fold in LOX, 1.2 ± 0.2-fold in LOXL-1, 4.9 ± 0.3-fold in LOXL-2, 2.8 ± 0.3-fold in LOXL-3, and 1.3 ± 0.1-fold in LOXL-4.
Significant increases of LOX family in ACL and MCL induced by TGF-β1 at different concentrations
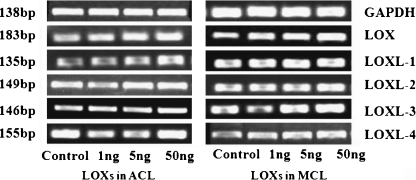
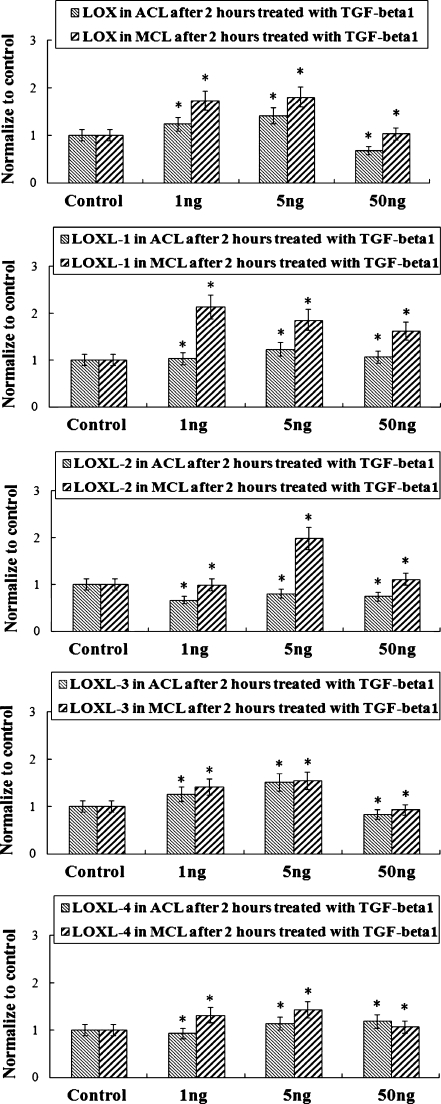
Figures 1 and 2 show that TGF-β1 up-regulated LOX gene profiles in ACL and MCL at different concentrations (1 ng/ml, 5 ng/ml, 50 ng/ml). Reversed transcript PCR showed elevated expression arrays in both ACL and MCL, and the elevated mRNA levels of LOXs in ACL was relatively lower than those in MCL. From real time quantitative PCR, after two hours of TGF-β1 treatments, we detected gene levels up to 123%, 141%, 68% (down-regulated to some extent, two hours treatment seems to have no break through) and 174%, 181%, 103% in LOX; 104%, 1.23%, 107% and 214%, 186%, 162% in LOXL-1; 125%, 151%, 84% and 141%, 154% , 93% in LOXL-3; 93%, 114%, 118% and 132%, 143%, 107% in LOXL-4 in ACL and MCL at 1 ng/ml, 5 ng/ml, and 50 ng/ml, respectively, compared with normal controls. LOXL-2 vary around control at two hours TGF-β1 treatments in both ACL and MCL. In addition, no correlation between DNA and age or sex was found.
Fig. 1.
Up-regulations of lysyl oxidase-like protein (LOXS) genes in both anterior cruciate ligament (ACL) and medial collateral ligament (MCL) after (TGF-β1) treatments with different concentrations by Reversed Transcript PCR. Through (GAPDH) (transforming growth factor-β1 glyceraldehyde phosphate dehydrogenase), the PCR arrays showed higher expressions of LOXs in MCL than these in ACL after treated with TGF-β1 (chose the concentrations of 1 ng, 5 ng, 50 ng). Size (in bp) of PCR products are indicated to the left of each pane
Fig. 2.
Elevated gene profiles of lysyl oxidase-like (LOX) proteins in anterior cruciate ligament (ACL) and medial collateral ligament (MCL) after TGF-β1 treatments with different concentrations by real-time quantitative PCR (mean ± SD) (n = 3). *Significant difference with respect to control (p < 0.05)
Five ng/ml TGF-β1 promoted strongly active gene expressions of LOXs in ACL and MCL, and an mRNA level peak appeared at different time points within 24 hours after treated with TGF-β1.
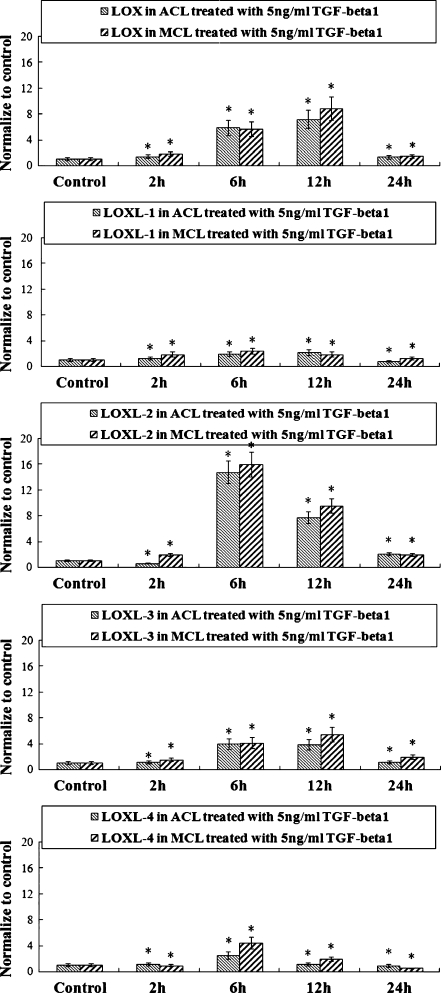
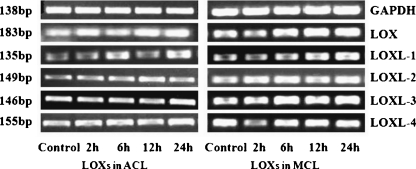
Because the time points at which a gene responds to TGF-β1 vary, we determined the mRNA levels of LOX family genes at zero, two, six, 12, and 24 hours after initiation of TGF-β1 treatments. We found that different LOXs mRNA levels peak at different time points. LOX and LOXL-3 reached peak at 12 hours, all others at six hours (see Fig. 3). Reversed transcript PCR arrays showed up-regulations of LOXs in both ACL and MCL, while elevated LOXs in ACL are relatively lower compared with those in MCL (Fig. 4).
Fig. 3.
Five ng/ml TGF-β1 promoted high expressions of lysyl oxidase-like (LOX) proteins in both anterior cruciate ligament (ACL) and medial collateral ligament (MCL), and elevated mRNA peaks appeared within 24 h after TGF-β1 treatments by real-time quantitative PCR (mean ± SD) (n = 3). An mRNA peak appeared at 12 h in LOX and LOXL-3; for LOXL-1, LOXL-2, and LOXL-4 the peak appeared at 6 h. *Significant difference with respect to control (p < 0.05)
Fig. 4.
Five ng/ml TGF-β1 up-regulated gene expressions of lysyl oxidase-like (LOX) proteins in both anterior cruciate ligament (ACL) and medial collateral ligament (MCL) by reversed transcript PCR. Through the expressions of GAPDH, the results showed higher expressions of LOXs in MCL than in ACL after 2 h treated with TGF-β1. Size (in bp) of PCR products are indicated to the left of each pane
LOXs showed different activities in ACL and MCL culture medium
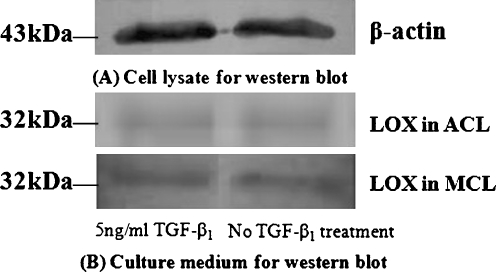
We performed Western blot analysis to show the expressions of protein levels of the LOX family in ACL and MCL after TGF-β1 treatments. We collected culture medium for LOX activity assay and cell lysate for β-actin activities. After 24 hours treated with 5 ng/ml TGF-β1, we collected supernatants of cultured ACL and MCL. Figure 5 shows the results of Western blots. The GS-800 (bio-rad) for Western blot in our lab showed the activities of sample medium treated with 5 ng/ml TGF-beta1 included a 10% increase compared with no treated control in ACL and 13% increase in MCL.
Fig. 5.
Expressions of lysyl oxidase-like (LOX) proteins in anterior cruciate ligament (ACL) and medial collateral ligament (MCL) by Western blot. After 16 h starvation in 2% FBS, the medium was switched to 1% FBS with 5 ng/ml TGF-β1 treatment. The culture medium samples were collected at 24 h for LOX assay, and cell lysate samples for activities of β-actin. Samples were subjected to Western blot
Discussion
The reparative capacity of ligament cells in animals and humans depends on their cellular properties and their response to growth factors [19]. The ACL is an extracellular structure surrounded by a thin layer of synovial tissue within an intra-articular environment such that when this synovial tissue is ruptured, the ACL is exposed to synovial fluids, haemorrhagic breakdown products and proteolytic enzymes [20]. Due to its extra-articular location and very limited vascular bed environment, the ACL is incapable of forming intermediate scar tissue and lacks an initial inflammatory response, resulting in poor tissue healing [21]. Compared with MCL, the level of pro-collagen mRNA are obviously lower in ACL at normal and injury states [22]. The ACL also has a poor vascular response after injury which makes its repair more difficult [23]. These intrinsic differences from MCL fibroblasts could help to explain ACL poor healing ability.
However, ACL and MCL injury and repair processes are very complex. It was reported from the rabbit model that expression of TGF-β1 was increased in and around the wound site in the rabbit MCL seven days following surgical injury, while, in the ACL, these protein expressions were limited to the injury site periphery and were lower in intensity than in MCL injuries [24]. In humans, ACL exhibit poor cellular properties, as well as a low level of response to growth factors, in regard to DNA synthesis, compared with those in MCL cells [18]. Proper administration of TGF-β1 can improve ACL healing [25, 26].
LOX is a key enzyme in ECM maturation through catalysing an essential enzymatic step in collagen and elastin cross-linking [16]. LOX up-regulation inferred an important role in the wound healing mechanism. We believe this is the first time LOX family expressions have been detected in ACL and MCL after TGF-β1 treatments. Our results of up-regulated expressions of LOXs in ACL and MCL were consistent with those previously reported in the kidneys of hereditary nephrotic mouse [17] and human lung fibroblasts [18]. Interestingly, Shanley et al. reported that TGF-β1 could increase the enzymatic activity of LOX [27]. Our results not only showed the increasing activities of LOX in both ACL and MCL, but also showed LOX activities were stronger in MCL than that in ACL fibroblasts.
The four LOX-like family members have fulfilled the traditional roles of cross-linkage by their homologous catalytic capacities. Substrates of LOXs for catalysis are mainly type I, II and III collagen and elastin [14, 16], while the mechanism of different catalytic efficiencies and capacities of all members is unknown. Kim et al. [28] reported that neither deletion nor truncation of the N-terminal domains affected amine oxidase activity LOXL4, but not LOXL-2. Yu et al. [29] revealed that LOXL-3 gene expressions, enzymatic activities, and LOXL-3-mediated collagen cross-linking in ventricular stiffness and lymphocyte cells paralleled with LOX.
Kim et al. [30] reported TGF-β1 regulated LOXL-4 expression through binding of AP1 transcription factor to a conserved region of the promoter in human HCC cell lines. In ACL and MCL, our results showed TGF-β1, as a multi-functional cytokine, up-regulated all LOX family members. As the whole family members fulfilled cross-linkage in the ECM, we inferred the stabilisation of ECM was the synergistic effect of LOXs, and proper high levels of TGF-β1 could help to promote the cure of injured ACL.
We must note that our TGF-β1 treatments only partially mimic the factors applied to LOX expressions during ACL injury, and do not represent the real situation. It is well known that in the knee joint fluids, the levels of IL-1α, and TNF-α are all elevated. These factors may further modulate the level of LOXs released from ACL and other sources. In our study, we have only analysed the LOXs of ACL and MCL fibroblasts response to TGF-β1. In the actual injury state, all factors may exert the effect together to induce LOX expressions during the injury cascade.
In conclusion, in this study, the results showed significant increases in mRNA levels of LOX family members induced by TGF-β1. At 5 ng/ml concentration of TGF-β1, the gene expressions of LOXs showed most activity. In order to mimic the real surroundings after ACL injury, we must pay attention to synergistic effects of inflammatory factors and growth factors to make good experiments in vitro to approach the cure of injured ACL.
Acknowledgements
This study was supported by Project 111 (B06023, China), an OREF Award (USA) and by NIH AR45635 (USA).
References
- 1.Nebelung W, Wuschech H. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy. 2005;21(6):696–702. doi: 10.1016/j.arthro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Legnani C, Ventura A, Terzaghi C, et al. Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int Orthop. 2010;34(4):465–471. doi: 10.1007/s00264-010-0963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furumatsu T, Hachioji M, Saiga K, et al. (2010) Anterior cruciate ligament-derived cells have high chondrogenic potential. Biochem Biophys Res Commun 391(1):1142–1147 [DOI] [PubMed]
- 4.Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2008;27(2):243–248. doi: 10.1002/jor.20763. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Kratz G, Haegerstrand A, Stahle-Backdahl M. Collagenase expression is rapidly induced in wound edge keratinocytes after acute injury in human skin, persists during healing, and stops at re-epithelialization. J Invest Derm. 1995;104(4):479–483. doi: 10.1111/1523-1747.ep12605917. [DOI] [PubMed] [Google Scholar]
- 6.Tang Z, Yang L, Xue R, et al. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: Involvement of the p65 subunit of NF-kappa B. Wound Repair Regen. 2009;17(5):709–716. doi: 10.1111/j.1524-475X.2009.00529.x. [DOI] [PubMed] [Google Scholar]
- 7.Meaney Murray M, Rice K, Wright RJ, Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21(2):238–244. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Cheng X, Wang J, Wang Y, Shi B, Huang C, et al. Novel chitosan/collagen scaffold containing transforming growth factor-beta1 DNA for periodontal tissue engineering. Biochem Biophys Res Commun. 2006;344(1):362–369. doi: 10.1016/j.bbrc.2006.03.106. [DOI] [PubMed] [Google Scholar]
- 9.Shao H-J, Lee YT, Chen CS, et al. Modulation of gene expression and collagen production of anterior cruciate ligament cells through cell shape changes on polycaprolactone/chitosan blends. Biomaterials. 2010;31(17):4695–4705. doi: 10.1016/j.biomaterials.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem. 1995;270(13):7176–7182. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- 11.Saito H, Papaconstantinou J, Sato H, Goldstein S. Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem. 1997;272(13):8157–8160. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 12.Jang W, Hua A, Spilson SV, Miller W, Roe BA, Meisler MH. Comparative sequence of human and mouse BAC clones from the mnd2 region of chromosome 2p13. Genome Res. 1999;9(1):53–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Maki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20(7):493–496. doi: 10.1016/S0945-053X(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 14.Kagan HM, Li Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88(4):660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 15.Csiszar K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70(70):1–32. doi: 10.1016/S0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 16.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto Y, Uchio-Yamada K, Anan S, et al. Transforming growth factor-beta1 mediated up-regulation of lysyl oxidase in the kidneys of hereditary nephrotic mouse with chronic renal fibrosis. Virchows Arch. 2005;447(5):859–868. doi: 10.1007/s00428-005-0001-8. [DOI] [PubMed] [Google Scholar]
- 18.Roy R, Polgar P, Wang Y, et al. Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: interactions among TGF-beta, IL-1 beta, and prostaglandin E. J Cell Biochem. 1996;62(3):411–417. doi: 10.1002/(SICI)1097-4644(199609)62:3<411::AID-JCB11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293–298. doi: 10.1007/s007760050106. [DOI] [PubMed] [Google Scholar]
- 20.Barlow Y, Willoughby Pathophysiology of soft tissue repair. Brit Med Bull. 1992;48(3):698–711. doi: 10.1093/oxfordjournals.bmb.a072572. [DOI] [PubMed] [Google Scholar]
- 21.Marshak DR, Lukas TJ, Watterson DM. Drug–protein interactions: Binding of chlorpromazine to calmodulin, calmodulin fragments and related calcium binding proteins. Biochemistry. 1985;24(1):144–150. doi: 10.1021/bi00322a020. [DOI] [PubMed] [Google Scholar]
- 22.Abi Ezzi SS, Foulk RA, Harwood FL, et al. Decrease in fibronectin occurs coincident with the increased expression of its integrin receptor alpha5beta1 in stress-deprived ligaments. Iowa Orthop J. 1997;17:102–109. [PMC free article] [PubMed] [Google Scholar]
- 23.Arnoczky SP. Physiological principles of ligament injuries and healing. In: Scott WN, editor. Ligament and extensor mechanism injuries of the knee: diagnosis and treatment. St. Louis: CV Mosby; 1991. pp. 67–81. [Google Scholar]
- 24.Lee J, Harwood FL, Akeson WH, Amiel D. Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J. 1998;18:19–25. [PMC free article] [PubMed] [Google Scholar]
- 25.Okuizumi T, Tohyama H, Kondo E, Yasuda K. The effect of cell-based therapy with autologous synovial fibroblasts activated by exogenous TGF-beta1 on the in situ frozen-thawed anterior cruciate ligament. J Orthop Sci. 2004;9(5):488–494. doi: 10.1007/s00776-004-0810-7. [DOI] [PubMed] [Google Scholar]
- 26.Falah M, Nierenberg G, Soudry M, et al. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34(5):621–630. doi: 10.1007/s00264-010-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanley CJ, Gharaee-Kermani M, Sarkar R, et al. Transforming growth factor-beta 1 increases lysyl oxidase enzyme activity and mRNA in rat aortic smooth muscle cells. J Vasc Surg. 1997;25(3):446–452. doi: 10.1016/S0741-5214(97)70254-4. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y-M, Kim E-C, Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38(1):145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 29.Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48(1):98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
- 30.Kim DJ, Lee DC, Yang SJ, et al. Lysyl oxidase like 4, a novel target gene of TGF-beta1 signaling, can negatively regulate TGF-beta1-induced cell motility in PLC/PRF/5 hepatoma cells. Biochem Biophys Res Commun. 2008;373(4):521–527. doi: 10.1016/j.bbrc.2008.06.071. [DOI] [PubMed] [Google Scholar]