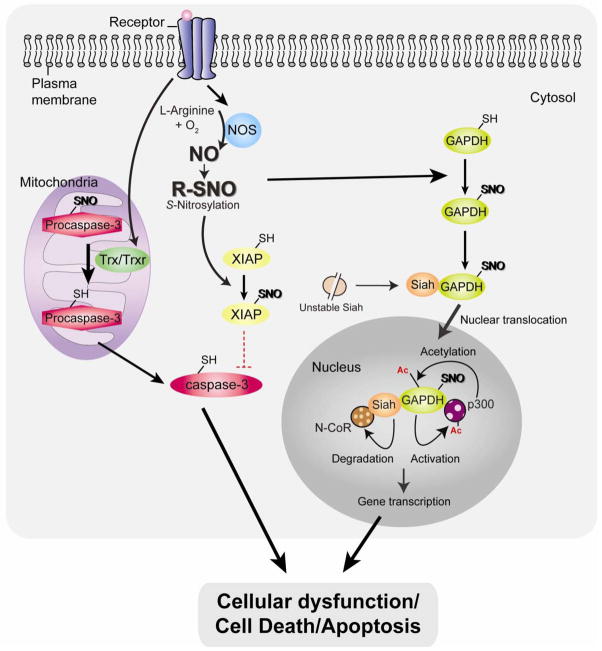
Fig. 2. Pro-cell death mechanisms associated with NO and protein S-nitrosylation: caspase activation and nuclear translocation of GAPDH.
Under stressed condition, activation of thioredoxin (Trx)/Trx reductase (TrxR) system (specifically in mitochondria) leads to procaspase3 denitrosylation, resulting in caspase-3 activation and apoptosis. In addition, NO inactivates the E3 ligase activity of X-linked inhibitor of apoptosis (XIAP) via S-nitrosylation (SNO), thus stabilizing caspases. Excessive nitrosative stress stimulates the formation of NO, which S-nitrosylates glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enabling it to bind and stabilize Siah. Siah’s nuclear localization signal mediates nuclear translocation of the GAPDH-Siah complex. Stabilized Siah in the protein complex with S-nitrosylated GAPDH facilitate degradation of nuclear co-repressor N-CoR. Nuclear translocated GAPDH is further acetylated at by the histone acetyltransferase p300 via direct protein interaction, which in turn stimulates the catalytic activity of p300. Both of these mechanisms by the nuclear GAPDH-Siah complex regulate gene expression, which results in cellular dysfunction/cell death/apoptosis.