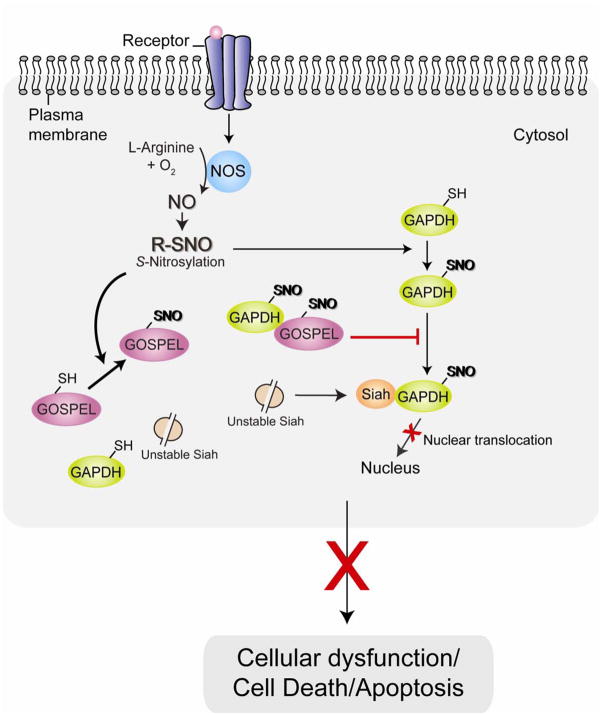
Fig. 3. GOSPEL as the regulator of the cell death mechanism associated with GAPDH nuclear translocation.
GOSPEL (GAPDH’s competitor Of Siah Protein Enhances Life) was recently identified as a physiological inhibitor against GAPDH-Siah binding. At the initial phase of nitrosative stress when NO levels are low, S-nitrosylation (SNO) of GOSPEL augments binding of GOSPEL and GAPDH, competing with Siah for binding to GAPDH, leading to retention of GAPDH in the cytosol and preventing its nuclear translocation and inhibiting cell death.