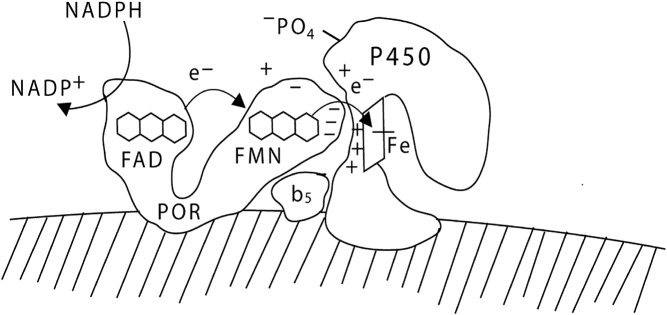
Fig. 2.
Electron transport to P450c17. POR, bound to the endoplasmic reticulum, receives a pair of electrons (e-) from NADPH and transfers them to its FAD moiety. Receipt of the electrons elicits a conformational change in POR, permitting the isoalloxazine rings of the FAD and FMN moieties to come close together so that the electrons pass from the FAD to the FMN. The POR then returns to its original orientation, permitting the FMN domain of POR to interact with the redox-partner binding site of P450c17. The electrons then travel through the P450c17 protein to reach the heme group and mediate catalysis. The interaction of POR and P450c17 is coordinated by negatively charged acidic residues on the electron-donating surface of the FMN domain of POR and positively charged basic residues in the concave redox-partner binding site of P450c17. The interaction of P450c17 and POR is facilitated by the allosteric action of cytochrome b5, and by the serine phosphorylation of P450c17. [© W. L. Miller.]