Abstract
Objectives:
The aim of the study was to compare the prevalence of vitamin D deficiency in obese and non-overweight children in North Texas, to examine relationships between dietary habits and 25-hydroxyvitamin D [25(OH)D] level in obese children, and to examine the relationship between 25(OH)D level and markers of abnormal glucose metabolism and blood pressure.
Patients and Methods:
Using a cross-sectional design, systolic and diastolic blood pressure, dietary information, serum 25(OH)D, fasting glucose and insulin, 2-h glucose from oral glucose tolerance test, hemoglobin A1c, and homeostasis model assessment of insulin resistance were recorded for 411 obese subjects (6–16 yr old) at an obesity referral clinic. 25(OH)D was also obtained from 87 control non-overweight subjects (6–16 yr old).
Results:
Ninety-two percent of obese subjects had a 25(OH)D level below 75 nmol/liter, and 50% were below 50 nmol/liter. Among non-overweight subjects, these frequencies were 68 and 22%, respectively (both P < 0.01 compared with obese subjects). 25(OH)D was negatively associated with soda intake (P < 0.001), juice intake (P = 0.009), and skipping breakfast (P < 0.001). 25(OH)D was negatively correlated with homeostasis model assessment of insulin resistance (r = −0.19; P = 0.001) and 2-h glucose (r = −0.12; P = 0.04) after adjustment for body mass index and age but was not correlated with hemoglobin A1c, systolic blood pressure Z score, or diastolic blood pressure Z score.
Conclusions:
Vitamin D deficiency is common in children in this southern United States location and is significantly more prevalent in obese children. Lower 25(OH)D level is associated with risk factors for type 2 diabetes in obese children.
The prevalence of obesity, defined as a body mass index (BMI) greater than the 95th percentile for age and sex (based on 1963 to 1994 norms), has tripled in U.S. children since 1980 and now describes 19% of children aged 6 through 19 yr (1). This rise in obesity prevalence has paralleled increases in childhood hypertension, hyperlipidemia, and type 2 diabetes (2). Childhood obesity is also associated with an increased prevalence of cardiovascular events and type 2 diabetes in adulthood (3).
High rates of vitamin D deficiency have been found in obese pediatric populations (4, 5). Furthermore, epidemiological data have linked low vitamin D levels to cardiovascular disease and type 2 diabetes (6, 7). The mechanisms by which obesity and its comorbidities are related to vitamin D deficiency are poorly understood.
In the present study, we determined the prevalence of vitamin D deficiency in children referred to an obesity clinic in North Texas and compared this prevalence to that of an age-, season-, and ethnicity-matched group of non-overweight children. We examined associations between 25-hydroxyvitamin D [25(OH)D] and dietary habits in obese children and tested whether there were correlations between 25(OH)D and markers of glucose homeostasis or blood pressure.
Subjects and Methods
Study population
In this cross-sectional study, 411 obese children (age, 6–16 yr; BMI, ≥95th percentile for age) were recruited consecutively from the Center for Obesity and its Consequences on Health (COACH) at Children's Medical Center Dallas (latitude, 33°N); 89 non-overweight children (age, 6–16 yr; BMI, <85th percentile for age) were matched to the obese group by season, age, and ethnicity. The non-overweight children were a convenience sample of otherwise healthy patients who were treated at the Endocrinology Center for congenital hypothyroidism, acquired primary hypothyroidism, or isolated GH deficiency.
Exclusion criteria for both obese and non-overweight children were: use of an anticonvulsant or systemic glucocorticoid; use of a vitamin D supplement more than 400 IU/d [400 IU is the current American Academy of Pediatrics recommendation for daily vitamin D intake for all children (8)]; hepatic disease, renal disease, or malabsorptive disorder; disorder of bone or calcium metabolism (including known vitamin D deficiency); hypothalamic disease; or genetic disorder that predisposes to obesity (such as Prader-Willi syndrome). Obese children taking an antihypertensive or glucose-lowering medication were included in the vitamin D deficiency prevalence and dietary analyses, but not in the correlation analysis of 25(OH)D to markers of glucose homeostasis and blood pressure. Non-overweight children with hypothyroidism were excluded if their free T4 levels were outside the normal range.
Data from obese children were collected from January 2009 to March 2010, and data from non-overweight children were collected from August 2009 to June 2010. The study was approved by the University of Texas Southwestern Medical Center Institutional Review Board. Written informed consent was obtained from the parent or guardian of all patients from whom data were collected prospectively, and assent was obtained from participants ages 10 and older.
Data collection
Age, sex, ethnicity, height, weight, and blood pressure were collected on all subjects. Ethnicity was self-assigned as Hispanic, African-American, Caucasian, or Other. Subjects designated as “Other” were not recruited into the non-overweight group due to the small number and heterogeneous nature of these subjects in the obese group. Age- and sex-specific BMI percentiles were determined from the 2000 Center for Disease Control growth charts, and percentiles were converted to Z scores (9). A medical assistant measured blood pressure in seated subjects with a Dinamap Procare Monitor (GE, Fairfield, CT). The average of the available measurements obtained from a single visit (up to three) was used for analysis. Systolic blood pressure (SBP) and diastolic blood pressure DBP were also converted into age- and sex-specific percentiles, which were converted to Z scores. BMI and blood pressure Z scores were used because these variables are age and gender dependent in children.
Subjects were classified according to the season in which their data were obtained: summer (June 21 to September 21), fall (September 22 to December 20), winter (December 21 to March 19), and spring (March 20 to June 20).
Dietary information obtained from obese subjects included average daily intake of 8-ounce glasses of soda, juice, and milk. Average daily fruit and vegetable intake was quantified as: less than one serving per day, one or two servings per day, or at least three servings every day. Breakfast intake was quantified as either “routinely skips breakfast” or “usually eats breakfast.”
Laboratory data included a serum 25(OH)D level in all subjects. In obese subjects, hemoglobin A1c (HgbA1c), fasting plasma glucose, and serum insulin were obtained, and an OGTT was performed. These are routine examinations in the COACH clinic. The OGTT was administered as follows: after the baseline fasting glucose and insulin were drawn, a standard flavored glucose dose (1.75 g/kg of body weight up to a maximum of 75 g) was given orally, and plasma glucose was drawn at 120 min. All blood samples were obtained between 0700 and 1200 h.
Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the formula: HOMA-IR = [(fasting insulin in μU/ml) × (fasting glucose in mg/dl)]/405.
Serum 25(OH)D was measured by chemiluminescent immunoassay (ARUP Laboratories, Salt Lake City, UT). The intraassay coefficient of variation was 6% at 30.8 nmol/liter and 4% at 90.5 nmol/liter, and the interassay coefficient of variation was 8% at 31.0 nmol/liter and 6% at 90.5 nmol/liter. HgbA1c was determined using the DCA Vantage Analyzer (Siemens, Tarrytown, NY). Plasma glucose was measured on the Siemens Dimension RXL automated chemistry analyzer by colorimetric reaction. Serum insulin was measured on the Siemens Immulite 2000 automated analyzer using a solid-phase, enzyme-labeled chemiluminescent immunometric assay.
Statistical analysis
The prevalence of vitamin D insufficiency and deficiency were determined for the obese and non-overweight study population and within each ethnic group and season. The following definitions of vitamin D deficiency are used in this study: sufficiency, 25(OH)D of at least 75 nmol/liter (30 ng/ml); insufficiency, less than 75 nmol/liter (30 ng/ml); and deficiency, less than 50 nmol/liter (20 ng/ml). A χ2 test was used to compare the prevalence rate of vitamin D insufficiency and deficiency in the obese group to the non-overweight group. Logistic regression models were used to estimate odds ratios of having vitamin D insufficiency and deficiency in the obese vs. non-overweight group adjusted for age, season, and ethnicity. Two-way ANOVA was used to compare 25(OH)D levels in the obese group to the non-overweight group, compare seasonal levels, and assess the interaction between obesity and season. Gender and ethnicity were also assessed with two-way ANOVA models.
In obese children, Pearson correlation coefficients were used to evaluate the relationship between 25(OH)D and 2-h glucose, HgbA1c, HOMA-IR, and SBP and DBP Z score. Data were adjusted for BMI Z score and age. When data did not meet tests of normality, data were log-transformed or non-parametric tests were used. An α of 0.05 was considered significant for all statistical tests. Analyses were conducted with SAS version 9.2 (SAS Institute, Inc., Cary, NC). Simple linear regression and multiple stepwise logistic regression analysis was performed for dietary data in relation to 25(OH)D using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA).
Results
Demographic characteristics of the groups are presented in Table 1. The age, season, and gender make-up of the obese and non-overweight groups were similar, but we were not able to achieve perfect matching. Serum 25(OH)D decreased with increasing age in all subjects (r = −0.29; P < 0.001). Average 25(OH)D levels did not differ by gender within the obese or non-overweight groups.
Table 1.
Clinical characteristics of study subjects
| Obese | Non-overweight | P | |
|---|---|---|---|
| n | 411 | 87 | |
| Age (yr) | 11.7 ± 2.6 | 11.2 ± 3.0 | 0.19 |
| Gender (% female) | 57 | 68 | 0.07 |
| BMI Z score | 2.4 (2.2 to 2.7) | 0.1 (−0.6 to 0.6) | |
| BMI percentile | 99.2 (98.6 to 99.6) | 53.3 (27.7 to 71.4) | |
| Ethnicity (%) | |||
| Hispanic | 49 | 56 | 0.19 |
| African-American | 27 | 13 | 0.005 |
| Caucasian | 21 | 31 | 0.05 |
| Other | 3 | 0 | |
| Season (%) | |||
| Spring | 32 | 26 | 0.34 |
| Summer | 26 | 21 | 0.34 |
| Fall | 17 | 28 | 0.02 |
| Winter | 26 | 25 | 0.92 |
| HOMA-IR | 5.2 (3.6 to 7.5)a | N/A | |
| 2-h Glucose (mg/dl) | 109 (95 to 127)b | N/A | |
| Hemoglobin A1c (%) | 5.5 (5.3 to 5.7)c | N/A | |
| SBP (mm Hg) | 117 (111 to 125)d | 106.5 (100 to 113) | |
| SBP Z score | 1.0 (0.4 to 1.7)d | 0.5 (−0.1 to 1.2) | |
| DBP (mm Hg) | 66 (61 to 71)d | 58 (55 to 63) | |
| DBP Z score | 0.3 (−0.2 to 0.7)d | −0.1 (−0.5 to 0.3) |
Values are presented as mean ± sd or median (interquartile range). N/A, Not available.
n = 327. The normal mean (95% CI) value for HOMA-IR in nonobese adolescent subjects is 2.30 (2.21–2.39) (32).
n = 298. Impaired glucose tolerance is defined as a 2-h glucose value above 140 mg/dl.
n = 384. The optimal (minimal misclassification) critical value of HgbA1c for screening for type 2 diabetes using our clinic's methodology is 5.7 (33).
n = 406.
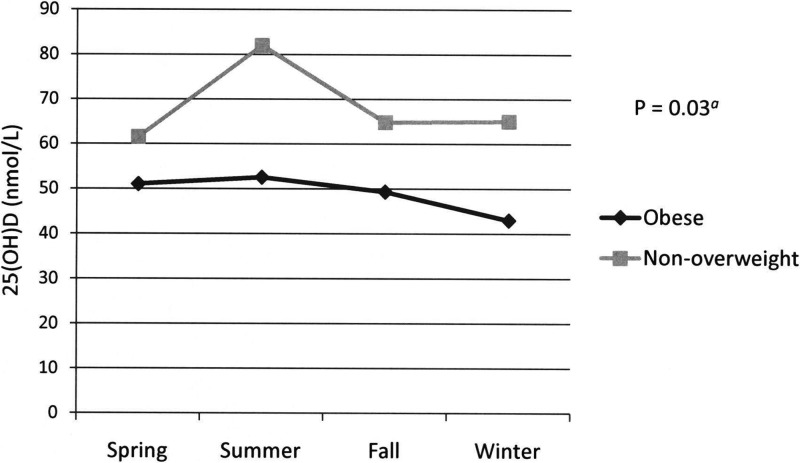
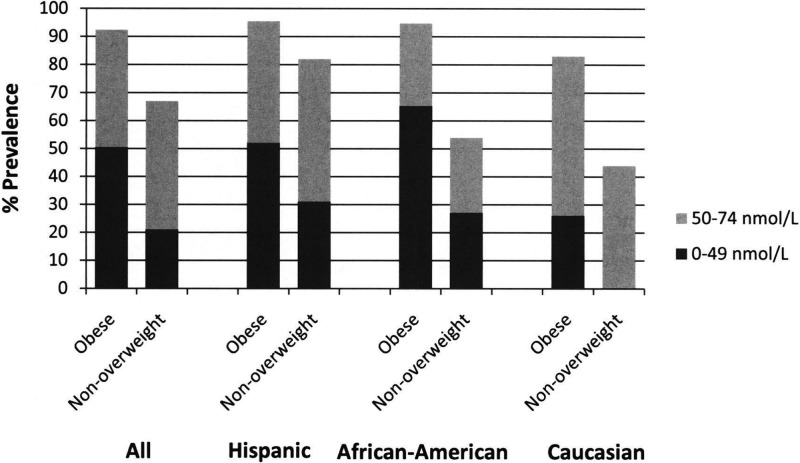
The mean serum 25(OH)D level was significantly lower in the obese group, for the entire cohort, within each ethnic group, and across all seasons (Table 2 and Fig. 1). Higher proportions of the obese group were vitamin D insufficient [25(OH)D level <75 nmol/liter] or deficient (<50 nmol/liter) than the non-overweight group (Fig. 2). There was significantly more seasonal variation of 25(OH)D level in the non-overweight group than in the obese group (P = 0.03) (Fig. 1).
Table 2.
Serum 25(OH)D (nmol/liter) concentration by gender, ethnicity, and season
| Obese |
Non-overweight |
P valueb | |||
|---|---|---|---|---|---|
| n | 25(OH)Da | n | 25(OH)Da | ||
| All | 411 | 49.0 ± 17.8 | 87 | 67.5 ± 26.8 | <0.0001 |
| Gender | |||||
| Male | 177 | 50.0 ± 17.3 | 28 | 73.3 ± 32.5 | <0.0001 |
| Female | 234 | 48.3 ± 18.0 | 59 | 64.8 ± 23.5 | <0.0001 |
| Ethnicity | |||||
| Hispanic | 200 | 47.8 ± 16.0 | 49 | 61.5 ± 27.3 | <0.0001 |
| African-American | 112 | 43.8 ± 18.0 | 11 | 66.3 ± 27.5 | 0.0002 |
| Caucasian | 88 | 58.0 ± 18.3 | 27 | 79.0 ± 22.8 | <0.0001 |
| Other | 11 | 51.8 ± 14.3 | N/A | N/A | |
| Season | |||||
| Spring | 130 | 51.0 ± 16.8 | 23 | 61.5 ± 23.0 | 0.01 |
| Summer | 105 | 52.5 ± 17.5 | 18 | 82.0 ± 32.5 | <0.0001 |
| Fall | 70 | 49.3 ± 17.8 | 24 | 64.8 ± 22.5 | 0.0007 |
| Winter | 106 | 43.0 ± 17.8 | 22 | 65.0 ± 25.3 | <0.0001 |
N/A, Not available.
25(OH)D values are expressed as mean ± sd.
P value for comparison between obese and non-overweight groups using two-way ANOVA.
Fig. 1.
Serum 25(OH)D concentration in obese vs. non-overweight subjects by season. Error bars denote sem; error bars are smaller than the symbols for the obese group. P = 0.03 for interaction of group and season by ANOVA of 25(OH)D values.
Fig. 2.
Prevalence of 25(OH)D deficiency by ethnicity.
The definitions for vitamin D deficiency used in this study differ from those recommended by the recent Institute of Medicine report on dietary reference intakes for calcium and vitamin D (10), which was published after our data collection and analysis was completed. This report defined vitamin D deficiency as 25(OH)D less than 30 nmol/liter (12 ng/ml) and inadequate vitamin D as 30 to 50 nmol/liter (12 to 20 ng/ml). Using the Institute of Medicine definition of vitamin D deficiency, 52 of 411 (12.7%) subjects in the obese population and three of 87 (3.4%) in the non-overweight population were vitamin D deficient.
The odds ratio for vitamin D deficiency in obese children compared with non-overweight children was 4.0 [95% confidence interval (CI), 2.3–6.9]. Adjusted for ethnicity, season, and age, the odds ratio was 3.6 (95% CI, 2.0–6.6). The odds ratio for vitamin D insufficiency in obese children compared with non-overweight children was 5.9 (95% CI, 3.3–10.5). Adjusted for ethnicity, season, and age, the odds ratio was 6.3 (95% CI, 3.3–12.0).
Dietary data are presented in Table 3. A total of 134 (33%) obese subjects reported that they routinely skipped breakfast. The two strongest predictors of decreased vitamin D levels were skipping breakfast (P < 0.001) and soda intake (P < 0.001). Milk intake was inversely correlated to 25(OH)D (P = 0.004) but was not independent of soda/juice intake. Taken together, skipping breakfast, soda intake, and juice intake predicted 25(OH)D level with an R2 of 0.106. Of these dietary habits, only skipping breakfast was correlated with age (older children more likely to skip breakfast; P < 0.0001). Thus, age (r2 = 0.094) and these three dietary habits were independent predictors of 25(OH)D levels (total R2, 0.172).
Table 3.
Variables predicting serum 25(OH)D in obese subjects
| Variable | n | Simple linear regression |
Multiple stepwise regression |
||||
|---|---|---|---|---|---|---|---|
| β | r2 | P | β | r2 | P | ||
| Age | 411 | −2.18 | 0.094 | <0.001 | −1.89 | 0.098 | <0.001 |
| Soda intake | 395 | −3.49 | 0.041 | <0.001 | −3.14 | 0.132 | |
| Skip breakfast | 411 | −8.66 | 0.053 | <0.001 | −5.62 | 0.158 | |
| Juice intake | 395 | −2.40 | 0.022 | <0.001 | −2.00 | 0.172 | |
| Milk intake | 395 | 3.28 | 0.023 | 0.002 | |||
| Fruit/vegetable intake | 405 | 3.82 | 0.021 | 0.003 | |||
| BMI Z score | 411 | −3.00 | 0.003 | 0.273 | |||
Skip breakfast, Yes/no; soda/juice/milk intake, number of 8-ounce glasses/day; fruit/vegetable intake, less than one, one or two, or at least three servings per day; β, regression coefficient.
The simple linear regressions are for the individual independent variables indicated. For the multiple stepwise regression analysis, only the four independent variables incorporated into the model are listed, and the r2 values displayed are cumulative [i.e. the four variables together account for ∼17% of the total variance in 25(OH)D levels]. The r2 for the three dietary variables (with age ignored) is 0.106.
In the obese group, there was a significant inverse relationship between 25(OH)D and HOMA-IR and weaker inverse correlations with 2-h glucose levels after OGTT, SBP Z score, and HgbA1c (Table 4). After adjustment for BMI Z score and age, the correlations with HOMA-IR and 2-h glucose remained statistically significant.
Table 4.
Relationship between serum 25(OH)D and markers of glucose homeostasis and blood pressure by Spearman correlation in obese subjects
| Variable | n | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|
| r | P | R | P | ||
| HOMA-IR | 327 | −0.27 | <0.0001 | −0.19 | 0.001 |
| 2-h Glucose | 298 | −0.14 | 0.02 | −0.12 | 0.04 |
| HgbA1c | 384 | −0.11 | 0.04 | −0.07 | 0.18 |
| SBP | 406 | −0.10 | 0.04 | −0.07 | 0.14 |
| DBP | 406 | 0.01 | 0.77 | −0.01 | 0.86 |
Statistical analysis was conducted using partial correlation, adjusted for age and BMI Z score.
Discussion
Our study compares serum 25(OH)D levels in obese and non-overweight children in North Texas, examines the relationship between dietary habits and serum 25(OH)D level, and examines correlations between 25(OH)D level and markers of glucose homeostasis and blood pressure in obese children.
25(OH)D and obesity
Studies have consistently shown that low 25(OH)D levels are associated with obesity in adults (11–16). Dong et al. (17) found that 25(OH)D levels were inversely related to BMI, waist circumference, total fat mass, and percentage of fat mass in a group of adolescents in Augusta, Georgia. Two recent studies reported high rates of vitamin D deficiency in obese pediatric populations [55% 25(OH)D <50 nmol/liter in New York, and 32% 25(OH)D <50 nmol/liter in Wisconsin] (4, 5). The prevalence of vitamin D deficiency in obese children in our study (50%) was similar to that seen in New York, but higher than that seen in Wisconsin, despite our more southern latitude. This difference is possibly explained by the smaller percentage of Caucasian subjects in our study population; 21% of our obese population was Caucasian compared with 39% in the Wisconsin study.
Data from the National Health and Nutrition Examination Survey (NHANES) 2001–2004 showed that obese children were more likely than non-obese children to have a 25(OH)D level below 37.5 nmol/liter [adjusted odds ratio, 1.9 (95% CI, 1.5–2.5)] (18). The odds ratios comparing vitamin D deficiency among obese vs. non-overweight children in our study [3.6 for a 25(OH)D level <50 nmol/liter, and 6.3 for a 25(OH)D level <75 nmol/liter] were markedly higher. One explanation for this difference is that our obese group was recruited from an obesity referral center and represents children at the far end of the obesity spectrum (the median BMI percentile of our obese group was 99.2%), whereas a BMI below the 85th percentile was required for inclusion to our non-overweight group (the median BMI percentile of our non-overweight group was 53.3%). We therefore compared groups with a wide difference in BMI percentile, which likely produced a larger difference in prevalence of vitamin D deficiency.
Several possibilities have been suggested to explain the lower 25(OH)D levels seen in obesity, including decreased sun exposure due to sedentary lifestyle, poor diet, and increased clearance of 25(OH)D due to storage in adipose tissue (19). The associations in our study between decreased 25(OH)D and soda intake and skipping breakfast have not, to our knowledge, been previously reported, and they lend support to poor dietary habits playing a role in lower 25(OH)D levels seen in obese children. It is noteworthy that such effects are apparently independent of any association between these dietary habits and obesity per se because there was no correlation between any of these dietary habits and BMI Z score in our patient population. The association with soda and juice intake suggests that children may be consuming soda and juice at the expense of milk. Milk is routinely fortified with 100 IU of vitamin D3 per 8-ounce serving, and it is one of the few dietary sources of vitamin D regularly consumed by children. Milk is often consumed at breakfast, and some breakfast cereals are fortified with vitamin D3, which may explain the association between skipping breakfast and low 25(OH)D. Adult data from the 1988–1994 NHANES showed a positive relationship between consumption of breakfast cereal more than three times per week and 25(OH)D level (20), and milk consumption has been associated with higher 25(OH)D levels in previous studies (21). The relationship between milk and 25(OH)D level in our study was significant, but not independent of soda and juice intake.
Serum 25(OH)D rises by 1.5 to 3 nmol/liter for every additional daily input of 100 IU cholecalciferol (22). In our study, the difference in mean 25(OH)D levels between obese and non-overweight subjects was 18.5 nmol/liter (67.5 vs. 49.0 nmol/liter). Obese children would need to consume an extra 600 to 1200 IU (the equivalent of six to 12 8-ounce cups of milk) of cholecalciferol daily to make up the difference in mean 25(OH)D levels seen in our study. Thus, poor dietary habits alone cannot explain the low 25(OH)D levels seen in obese children.
In our study, non-overweight children had greater seasonal variations in 25(OH)D levels than did obese children (Fig. 1). Greater sun exposure in non-overweight children or increased 25(OH)D sequestration in the adipose tissue of obese children could potentially explain this seasonal difference. It is noteworthy that BMI Z score did not predict 25(OH)D levels within this already very obese group of children. Fat mass might have been a better predictor, but body composition data were not available to permit calculation of this parameter. There is not an obvious explanation for the observed decrease of 25(OH)D levels with age, given that this association was independent of BMI and dietary habits; age-related changes in unmeasured factors such as sun exposure, fat mass, or other dietary habits might explain this observation.
25(OH)D and type 2 diabetes
Since the description of impaired pancreatic insulin secretion in vitamin D-deficient rats 30 yr ago (23), many observational studies have investigated the relationship between vitamin D status and type 2 diabetes (6). Many, but not all, show that lower 25(OH)D levels are associated with a higher prevalence of impaired glucose tolerance or type 2 diabetes.
In the 1988–1994 NHANES, adult non-Hispanic whites and Mexican-Americans in the highest 25(OH)D quartile had a significantly lower odds of diabetes than those in the lowest 25(OH)D quartile (24). 25(OH)D was positively correlated with the quantitative insulin-sensitivity check index (QUICKI, a measure of insulin sensitivity) and negatively correlated with HgbA1c among 127 obese children (aged 6–17 yr) in Wisconsin (4). In the 2001–2004 NHANES, children (aged 12–19 yr) in the lowest 25(OH)D quartile had an adjusted odds ratio of 2.54 (95% CI, 1.01–6.40) for fasting hyperglycemia compared with those in the highest quartile (25).
Potential explanations for the association between low 25(OH)D level and impaired glucose tolerance have largely focused on direct effects of vitamin D on pancreatic β-cell insulin secretion. Vitamin D receptors and vitamin D-binding proteins are known to exist in pancreatic tissue, and calcium plays an essential role in β-cell insulin secretion (26, 27). In our study, we were unable to directly test the relationship between 25(OH)D and insulin secretion. We did find, however, a significant inverse relationship between 25(OH)D and HOMA-IR, a reliable surrogate for insulin resistance (28). This relationship to insulin resistance is consistent with associations seen in previous studies. Mechanistic links between vitamin D and insulin sensitivity have been proposed. A vitamin D response element in the insulin receptor gene promoter might affect insulin receptor expression (29); 1,25-dihydroxyvitamin D3, the active form of vitamin D, induces increased transcription and protein expression of the insulin receptor in in vitro studies (30).
A potential limitation to our study is the control group of non-overweight children that was recruited from a convenience sample of patients followed in our endocrinology clinic. We are not aware of any relationship between the diagnoses of our control subjects (treated congenital or acquired hypothyroidism and treated isolated GH deficiency) and 25(OH)D status. In addition, the 25(OH)D status of our control group is similar to that of a recent large, nationally representative sample. NHANES (2001 to 2006) reported that among 4558 children 1 to 11 yr old, the mean serum 25(OH)D level was 68.0 nmol/liter, compared with the mean serum 25(OH)D level in our non-overweight group of 67.5 nmol/liter (31).
We were not able to measure physical activity or sunlight exposure in either the obese or non-overweight group. Comparison of this information between the two groups would have been helpful in further understanding the mechanism(s) behind the difference in 25(OH)D status between the two groups.
As the number of obese children increases, pediatric providers must be aware of the higher rates of vitamin D deficiency seen in obese vs. non-overweight children. Lifestyle factors such as skipping breakfast, increased soda intake, and increased juice intake appear to contribute to the lower 25(OH)D levels seen in obese children. In our study, 25(OH)D levels in obese children were negatively correlated with both insulin resistance (as measured by HOMA-IR) and 2-h glucose levels from an OGTT. Thus, low 25(OH)D levels may play a role in the pathophysiology of impaired glucose tolerance in obese children.
Future studies are needed to determine the clinical sequelae of lower 25(OH)D levels in obese children, the amount and duration of treatment necessary to replenish 25(OH)D in these children, and whether treatment with vitamin D can improve primary clinical endpoints such as insulin resistance.
Acknowledgments
We thank Beverley Huet for statistical expertise and review of this manuscript.
The authors received support from National Institutes of Health Clinical and Translational Science Awards Grant UL1 RR024982 in the form of statistical assistance.
This work was presented, in part, at the 2010 Pediatric Academic Societies' Annual Meeting held in Vancouver, British Columbia, Canada, May 1–4, 2010.
Disclosure Summary: The authors declare they have no conflict of interest.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- DBP
- diastolic blood pressure
- HgbA1c
- hemoglobin A1c
- HOMA-IR
- homeostasis model assessment of insulin resistance
- OGTT
- oral glucose tolerance test
- 25(OH)D
- 25-hydroxyvitamin D
- SBP
- systolic blood pressure.
References
- 1. Ogden CL, Carroll MD, Flegal KM. 2008. High body mass index for age among US children and adolescents, 2003–2006. JAMA 299:2401–2405 [DOI] [PubMed] [Google Scholar]
- 2. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. 1999. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 103:1175–1182 [DOI] [PubMed] [Google Scholar]
- 3. Dietz WH. 1998. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 101:518–525 [PubMed] [Google Scholar]
- 4. Alemzadeh R, Kichler J, Babar G, Calhoun M. 2008. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 57:183–191 [DOI] [PubMed] [Google Scholar]
- 5. Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. 2007. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab 20:817–823 [DOI] [PubMed] [Google Scholar]
- 6. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. 2007. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holick MF. 2004. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80(6 Suppl):1678S–1688S [DOI] [PubMed] [Google Scholar]
- 8. Wagner CL, Greer FR. 2008. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122:1142–1152 [DOI] [PubMed] [Google Scholar]
- 9. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. 2002. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60 [DOI] [PubMed] [Google Scholar]
- 10. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. 2011. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizzoli R, Eisman JA, Norquist J, Ljunggren O, Krishnarajah G, Lim SK, Chandler J. 2006. Risk factors for vitamin D inadequacy among women with osteoporosis: an international epidemiological study. Int J Clin Pract 60:1013–1019 [DOI] [PubMed] [Google Scholar]
- 12. Bischof MG, Heinze G, Vierhapper H. 2006. Vitamin D status and its relation to age and body mass index. Horm Res 66:211–215 [DOI] [PubMed] [Google Scholar]
- 13. Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. 1988. Low circulating vitamin D in obesity. Calcif Tissue Int 43:199–201 [DOI] [PubMed] [Google Scholar]
- 14. Looker AC. 2005. Body fat and vitamin D status in black vs. white women. J Clin Endocrinol Metab 90:635–640 [DOI] [PubMed] [Google Scholar]
- 15. Arunabh S, Pollack S, Yeh J, Aloia JF. 2003. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 88:157–161 [DOI] [PubMed] [Google Scholar]
- 16. Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. 2009. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res 29:3713–3720 [PubMed] [Google Scholar]
- 17. Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, Keeton D, Petty K, Holick MF, Zhu H. 2010. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics 125:1104–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. 2009. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124:e362–e370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. 2000. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693 [DOI] [PubMed] [Google Scholar]
- 20. Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. 2002. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76:187–192 [DOI] [PubMed] [Google Scholar]
- 21. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. 2004. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531–537 [DOI] [PubMed] [Google Scholar]
- 22. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. 2003. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204–210 [DOI] [PubMed] [Google Scholar]
- 23. Norman AW, Frankel JB, Heldt AM, Grodsky GM. 1980. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 209:823–825 [DOI] [PubMed] [Google Scholar]
- 24. Scragg R, Sowers M, Bell C. 2004. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 27:2813–2818 [DOI] [PubMed] [Google Scholar]
- 25. Reis JP, von Mühlen D, Miller ER, 3rd, Michos ED, Appel LJ. 2009. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 124:e371–e379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee S, Clark SA, Gill RK, Christakos S. 1994. 1,25-Dihydroxyvitamin D3 and pancreatic β-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 134:1602–1610 [DOI] [PubMed] [Google Scholar]
- 27. Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, Fleischer N, Sharp GW, Christakos S. 1999. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and βcell lines. J Biol Chem 274:34343–34349 [DOI] [PubMed] [Google Scholar]
- 28. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. 2000. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23:57–63 [DOI] [PubMed] [Google Scholar]
- 29. Maestro B, Dávila N, Carranza MC, Calle C. 2003. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 84:223–230 [DOI] [PubMed] [Google Scholar]
- 30. Maestro B, Campión J, Dávila N, Calle C. 2000. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 47:383–391 [DOI] [PubMed] [Google Scholar]
- 31. Mansbach JM, Ginde AA, Camargo CA., Jr 2009. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 124:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. 2006. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care 29:2427–2432 [DOI] [PubMed] [Google Scholar]
- 33. Shah S, Kublaoui BM, Oden JD, White PC. 2009. Screening for type 2 diabetes in obese youth. Pediatrics 124:573–579 [DOI] [PubMed] [Google Scholar]