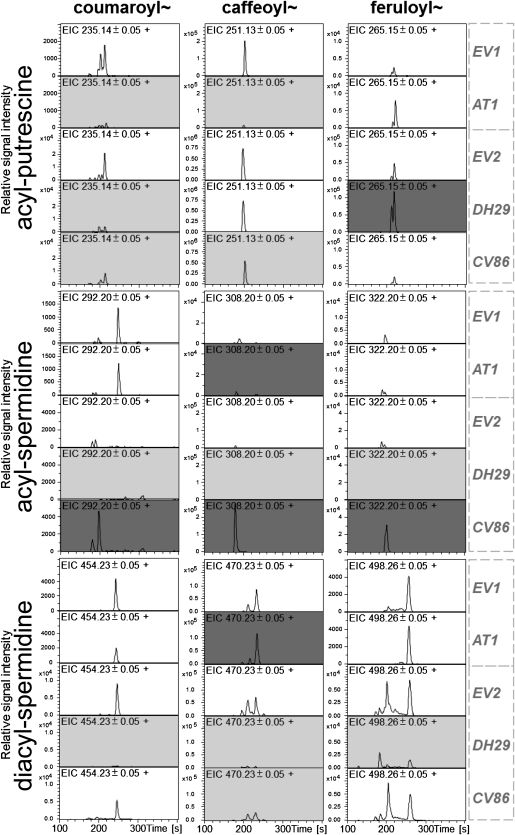
Figure 4.
Representative (n = 4) extracted ion chromatograms (EIC) for monoacylated putrescines and monoacylated and diacylated spermidines in 4-d herbivory-elicited leaves of EV plants and AT1-, DH29-, and CV86-silenced plants. Ion types selected for the calculation of EIC traces correspond to the protonated [M+H]+ m/z signals of the different isomers as follows: (first row, left to right), N-coumaroylputrescine, N-caffeoylputrescine, N-feruloylputrescine; (second row), N-coumaroylspermidine, N-caffeoylspermidine, N-feruloylspermidine; (third row), N′,N″-coumaroyl,caffeoylputrescine, N′,N″-dicaffeoylspermidine, N′,N″-diferuloylspermidine. Indexes after nitrogen atoms indicate that structural rearrangements during in-source or collision-induced dissociation-MS/MS fragmentation did not allow the unequivocal assignment of the phenylpropanoid residues to the N1, N5, or N10 position of spermidine. Metabolites showing at least 1.5-fold reduced and increased content are highlighted using light gray and dark gray background, respectively (Supplemental Table S5). EV1 and EV2 show control treatments with an EV infiltration in two independent VIGS experiments conducted separately for AT1 (EV1) and DH29 + CV86 (EV2).