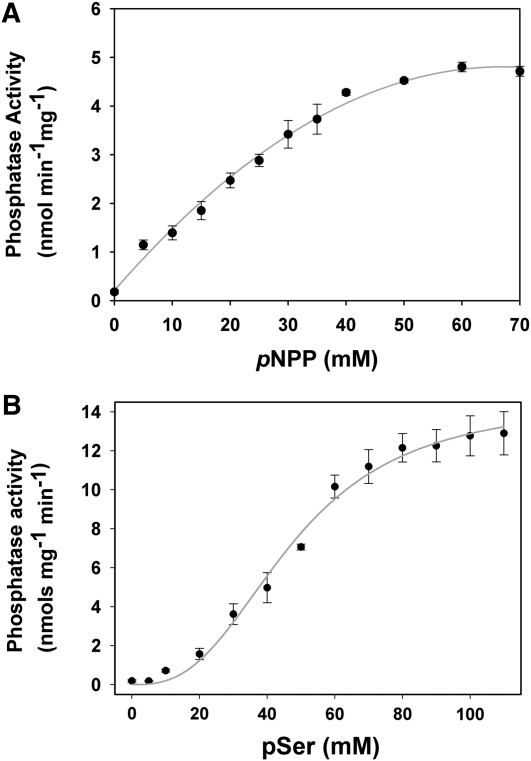
Figure 5.
Enzymatic kinetics of phosphatase activity with two different substrates, pNPP and pSer. A, Michaelis-Menten plot of AtTLP18.3 with pNPP used as the substrate. The Km for pNPP is 42.56 mm, and the Vmax is 8.11 nmol min−1 mg−1. B, Michaelis-Menten plot of AtTLP18.3 with pSer used as the substrate. The Km for pSer is 47.51 mm, and the Vmax is 14.55 nmol min−1 mg−1. The kinetics assay of AtTLP18.3 shows a classical hyperbolic saturation with pNPP but an allosteric sigmoid with pSer. Error bars represent se.