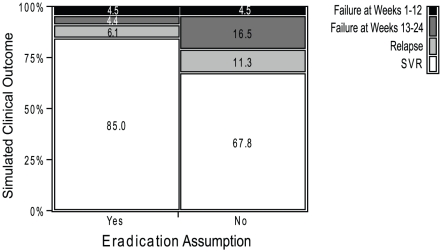
Figure 5. Predicted clinical outcome among treatment-naïve patients who completed T12PR24 treatment, with and without the eradication assumption.
Notes: The simulations are for a simulated treatment-naïve population, with HCV genotype 1a∶1b ratio of 1∶1. The analyses of sensitivities to the eradication assumption were performed as follows: “Yes”, if variants cannot replicate when their levels are below eradication limit; “No”, if variants can replicate when their levels are below eradication limit. The simulated clinical outcomes were defined as follows: Failure at Week 1–12, HCV RNA returns back to detectable levels in the first 12 weeks (during telaprevir treatment); Failure at Week 13–24, HCV RNA levels return back to detectable level during Weeks 13–24 of therapy (during PR treatment, after completion of 12 weeks of telaprevir treatment); Relapse, HCV RNA undetectable at the end of treatment, but did not reach eradication; SVR, eradicated prior to the end of treatment. Compared to the simulated outcomes without the eradication assumption, the simulated outcomes with the eradication assumption better matched the observed clinical outcomes.