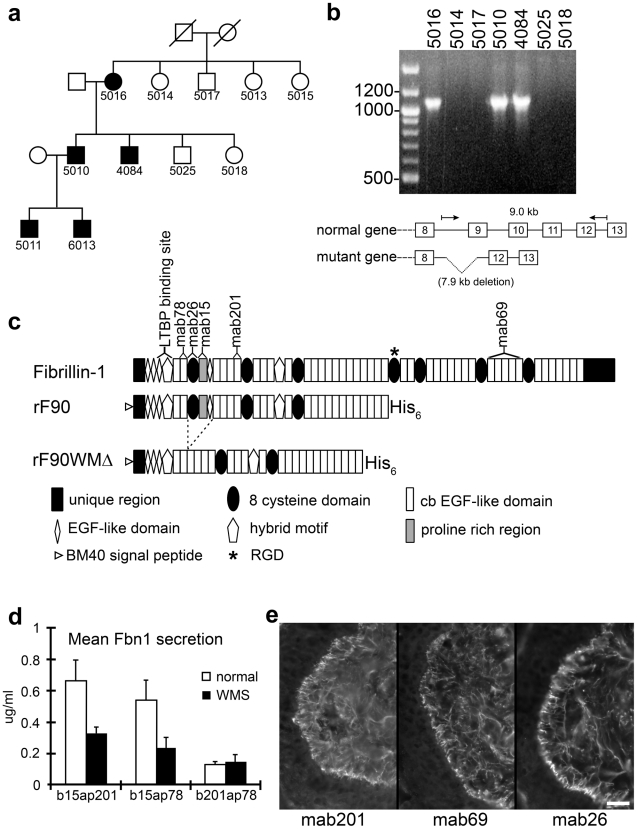
Figure 1. Characterization of a novel genomic deletion in FBN1 in a family with WMS.
(a) Partial pedigree of the family. Affected individuals are shown as filled symbols. (b) PCR from genomic DNA of selected family members, using primers flanking the deleted region. Only the affected individuals give rise to the shortened product (1.1 kb), which after DNA sequencing revealed a 7895 nt deletion with boundaries in FBN1 introns 8 (IVS8-1207) and 11 (IVS11+1257). The wildtype product (9.0 kb) was too large to be amplified under the conditions used. (c) Schematic drawing of domains contained in the recombinant N-terminal half of wildtype (rF90) and WMS deleted fibrillin-1 protein (rF90WMΔ). The mutation results in the deletion of exons 9–11, encoding the first 8-cysteine domain, the adjacent proline rich region, and a generic EGF-like domain. Purity of preparations of rF90 and rF90WMΔ is shown in Figure S3. Epitopes of monoclonal antibodies used in this study are shown above the full-length molecule. In addition, the single RGD site and the binding site for LTBP are marked. (d) Sandwich ELISA used to quantitate secretion of wildtype and mutant fibrillin-1 in culture medium from normal dermal fibroblasts and WMS fibroblasts. Capture antibodies were biotinylated (b), and detector antibodies were coupled to alkaline phosphatase (ap). The capture and detector antibody pair that recognizes epitopes outside of the deleted region (b201 and ap78) detected both normal and WMS mutant fibrillin molecules and showed equal levels of fibrillin-1 secretion in both wildtype and WMS fibroblast medium. When mAb15 was used as a capture antibody, only wildtype fibrillin-1 was detected, because the proline-rich region, which contains the epitope recognized by mAb15 [33] is deleted in WMS fibrillin-1. Quantitation of fibrillin-1 secretion using the b15ap201 and b15ap78 antibody pairs showed approximately half the amount of fibrillin-1 in WMS fibroblast medium compared to medium from normal fibroblasts. These results indicate that equal amounts of normal and mutated fibrillin-1 are secreted by WMS fibroblasts. The differences in total fibrillin-1 amounts measured using the different pairs are due to differences in affinities of the pairs for the protein standard (rF11). For this experiment, n = 3 and the error bars represent the standard deviation. (e) Immunofluorescence of WMS skin showed a normal fibrillin pattern when mAb201, mAb69, or mab26 were used. Unlike immunofluorescence of Marfan skin [10], fibrillin-1 staining patterns were not fragmented in WMS skin. Scale bar = 20 µm.