Abstract
Study Objective
Little is known about racial differences in androgen levels among obese children. The objective of this pilot study was to compare basal and stimulated androgen levels in a cross-sectional sample of obese black and white pubertal females.
Study Design, Setting, and Participants
This was cross-sectional study of obese (BMI ≥95th percentile) but otherwise healthy female adolescents (10 black and 12 white; age range 8.8–13.9 y) who underwent ACTH stimulation testing at an academic medical center as part of a protocol for the study of obesity-related conditions.
Main Outcome Measures
Basal and stimulated androgen levels.
Results
White and black participants were similar with regard to pubertal stage, BMI, percent body fat, fasting glucose and insulin levels. Black girls had lower stimulated levels of 17-hydroxyprogesterone and the differences between basal and stimulated levels of 17-hydroxyprogesterone and androstenedione were lower in black girls. BMI was negatively correlated with stimulated cortisol in blacks only (r = −0.69, p = 0.03).
Conclusion
There appear to be race-related differences in stimulated androgen levels in obese adolescent females. This is deserving of further study, as measurements of androgen levels are commonly used in clinical practice and research.
Keywords: ACTH stimulation test, race, cortisol, obesity, overweight, androstenedione, 17-hydroxyprogesterone, testosterone, 17-hydroxypregnenolone, dehydroepiandrosterone
INTRODUCTION
Measurement of basal and stimulated androgen levels is often performed during the evaluation of conditions related to obesity and insulin resistance, such as premature adrenarche and polycystic ovary syndrome. However, little is known about the clinical significance of potential racial differences in androgen levels among obese children. The objective of this pilot study was to compare basal and adrenocorticotropic (ACTH)-stimulated androgen levels in a cross-sectional sample of obese black and white pubertal females.
METHODS
The study was approved by the University of Pittsburgh Institutional Review Board and was performed in the Pediatric Clinical and Translational Research Center (PCTRC) at Children’s Hospital of Pittsburgh. After obtaining informed consent, 22 obese (BMI ≥95th percentile) but otherwise healthy female adolescents (10 black and 12 white; age range 8.8 – 13.9 y) participated in the collection of baseline measures prior to further treatment for obesity at the Weight Management & Wellness Center at Children’s Hospital of Pittsburgh. Power calculations indicate that at least 26 participants per group would be required to demonstrate a 30% difference between groups in stimulated androgen levels (based on estimates of a 100 ±35 ng/dL increase in 17 OH-Progesterone). However, this was a pilot study with limited resources allowing for analysis of data from 22 subjects to generate pilot data. Exclusion criteria were chronic diseases or medications which could interfere with endocrine function or glucose regulation, neurologic/psychiatric disorders, and syndromic obesity.
A fasting blood sample was obtained for measurement of baseline androgens (total and free testosterone, sex hormone binding globulin (SHBG); Esoterix, Inc.), insulin, and lipid profile. ACTH stimulation testing was performed by administering Cortrosyn (0.25 mg, iv) over 1 minute. Blood samples were obtained for 17-hydroxypregnenelone, 17-hydroxyprogesterone, androstenedione, and dehydroepiandrosterone (DHEA) before administration of Cortrosyn and 30 min post-infusion (1). Homeostasis model assessment-insulin resistance [HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L) divided by 22.5] was calculated to express basal insulin resistance. Body composition was measured by air displacement plethysmography (BOD POD; Life Measurement, Inc).
Two-sided t tests were used to compare continuous variables between black and white participants. Mann Whitney U tests were utilized for non-normally distributed outcomes. Pearson correlation coefficients were calculated to quantify the association between BMI and outcome variables after log transformation of non-normally distributed data, and partial correlations were calculated after controlling for race or BMI. Data are expressed as the mean ±SEM unless otherwise noted.
RESULTS
The anthropometric and metabolic characteristics of participants according to race are shown in Table 1. Age and pubertal stage (mean Tanner stage = 4±1 for breasts and pubic hair) did not differ between the groups. Three white and 2 black participants were post-menarchal. Testing was scheduled to target the follicular phase of the menstrual cycle; however, 1 black and 1 white participant had progesterone levels >200 ng/dL on the day of testing. BMI, percent body fat, fasting glucose, lipids, glycosylated hemoglobin (HbA1C), fasting insulin and C-peptide, HOMA-IR, total/free testosterone, and sex hormone binding globulin (SHBG) were not significantly different among white and black participants.
Table 1.
Anthropometric and metabolic characteristics of participants according to race
| White Girls (N=12) |
Black Girls (N=10) |
P | |
|---|---|---|---|
| Age (yr) | 11.8 ± 0.4 | 11.2 ± 0.5 | 0.36 |
| BMI (kg/m2) | 28.4 ± 1.1 | 31.2 ± 1.9 | 0.21 |
| BMI SDS | 2.30 ± 0.22 | 3.17 ± 0.35 | 0.42 |
| Percent body fat (%) | 35.6 ± 1.8 | 39.1 ± 2.5 | 0.24 |
| Fat mass (kg) | 25.5 ± 2.0 | 30.1 ± 4.9 | 0.37 |
| Lean mass (kg) | 46.3 ± 3.3 | 44.2 ± 3.3 | 0.67 |
| Triglycerides (mg/dL) | 121 ± 22 | 80 ± 7 | 0.10 |
| Cholesterol (mg/dL) | 141 ± 10 | 128 ± 8 | 0.35 |
| LDL (mg/dL) | 85 ± 8 | 79 ± 7 | 0.64 |
| HDL (mg/dL) | 37 ± 4 | 35 ± 2 | 0.75 |
| VLDL (mg/dL) | 19 ± 3 | 13 ± 1 | 0.12 |
| TG/HDL | 3.9 ± 0.8 | 2.4 ± 0.2 | 0.11 |
| Non-HDL cholesterol (mg/dL) | 104 ± 9.4 | 92 ± 7.7 | 0.37 |
| HbA1C (%) | 5.2 ± 0.1 | 5.3 ± 0.1 | 0.81 |
| Fasting glucose (mg/dL) | 87 ± 2.2 | 84 ± 1.7 | 0.36 |
| Fasting insulin (μU/mL)* | 34.7 ± 5.5 | 32.4 ± 5.1 | 0.74 |
| Fasting C-peptide (ng/mL)* | 3.07 ± 0.38 | 2.50 ± 0.40 | 0.32 |
| HOMA-IR* | 7.56 ± 1.34 | 6.72 ± 1.06 | 0.64 |
| Testosterone (ng/dL)* | 18.0 ± 4.2 | 20.9 ± 9.7 (n=9) | 0.80 |
| Free testosterone (pg/mL)* | 2.33 ± 0.49 | 3.29 ± 1.87 (n=9) | 0.67 |
| Percent free testosterone (%) | 1.37 ± 0.12 | 1.28 ± 0.14 (n=9) | 0.63 |
| SHBG (nmol/L) | 30.5 ± 4.1 | 26.3 ± 4.1 | 0.43 |
Data not normally distributed. Mann Whitney U test for non-parametric data was used to compare differences between groups.
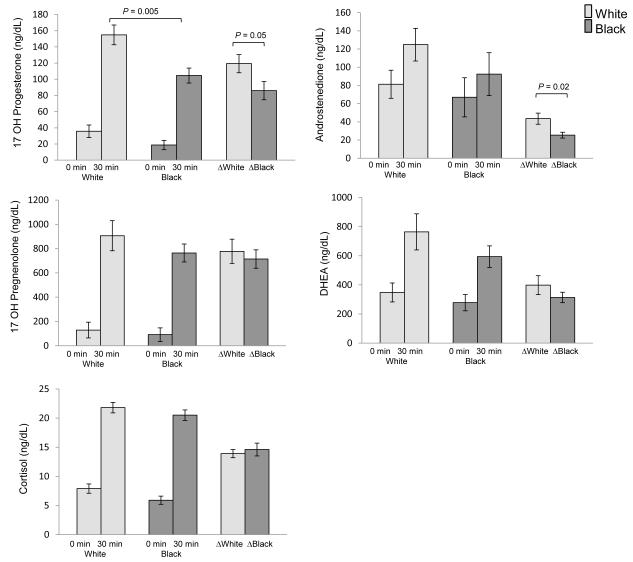
Baseline and stimulated adrenal hormone levels according to race are shown in Figure 1. Stimulated 17-hydroxyprogesterone levels were 32% lower in black girls. The response to ACTH stimulation in 17-hydroxyprogesterone (Δ 17-hydroxyprogesterone) was 28% lower in black girls, and Δ androstenedione was 42% lower in black compared with white girls. There were no significant differences according to race in baseline or stimulated cortisol, DHEA, or 17-hydroxypregnenelone.
Figure 1.
Baseline and stimulated adrenal steroid concentrations (Δ) during ACTH stimulation testing according to race (white n=12, black n=10). Fewer subjects had complete data for 17-hydroxypregnenolone (white n=12, black n=9) and DHEA (white n=11, black n=9).
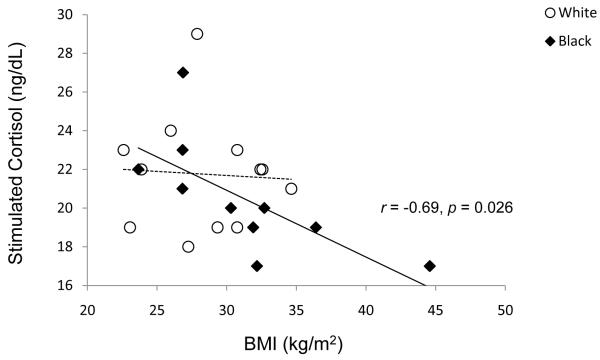
Stimulated cortisol and delta cortisol were negatively correlated with BMI (r = −0.434, p = 0.043 and r = −0.478, p = 0.025). After controlling for race, correlations with BMI were no longer significant for the study group as a whole (stimulated cortisol r = −0.369, p = 0.146; delta cortisol r = −0.397, p = 0.114). When the correlation analysis was performed separately for blacks and whites, BMI was negatively correlated with stimulated cortisol in blacks only (Figure 2, r = −0.6934, p = 0.026).
Figure 2.
The relationships between stimulated cortisol levels (30 min after the administration of ACTH) and BMI in black and white participants. The correlation between BMI and stimulated cortisol level was significant among blacks (solid line), but not whites (dashed line, r=−0.06, p=0.86).
Delta cortisol was negatively correlated with both fasting insulin (r = −0.478, p = 0.024) and HOMA-IR (r = −0.431, p = 0.045). SHBG was negatively correlated with fasting insulin (r = −0.434, p = 0.044). However, after controlling for BMI, these correlations were no longer significant. After controlling for race, SHBG was negatively correlated with BMI (r = −0.517, p = 0.034), and positively associated with stimulated androstenedione (r = 0.484, p = 0.049) for the study group as a whole. Neither BMI nor percent body fat were significantly correlated with other measures from the ACTH stimulation test.
DISCUSSION
The results of this study suggest that, in obese adolescent females, there are race-related differences in stimulated androgen levels. Black race was associated with lower stimulated levels of 17-hydroxyprogesterone and a smaller magnitude of increase 17-hydroxyprogesterone and androstenedione in response to ACTH. Stimulated cortisol levels were negatively associated with BMI in black females. Our results are in agreement with 1) studies in adults showing black women to have lower androstenedione levels than white women, despite black women having higher BMIs (2, 3); and 2) a cross-sectional survey of healthy children (n=360 girls; 6 – 18 yr), which showed that black girls had lower serum A.M. androstenedione levels than white girls with similar weights and Tanner staging (4). We are unaware of similar studies which have compared stimulated androgens in black and white women or children. The physiological significance of this racial difference in androstenedione is unknown, but differences in body fat distribution, insulin resistance, and genetic factors may be contributory.
We did not observe a significant relationship between measures of adiposity and androgen levels, likely because all of the participants in this study were obese. Other cohort studies have shown obese pre-pubertal and pubertal girls to have significant hyperandrogenemia and low SHBG, as compared with lean girls; and that weight loss promotes decreases in testosterone levels (5). In the present study the participants did not have clinical evidence of hyperandrogenism. Thus, characteristics of the obese but otherwise healthy study population may have decreased the apparent relationship between androgen excess and adiposity.
The relationship between measures of adrenocortical activity and adiposity among developing children is confounded by environmental and socioeconomic factors which also impact cortisol levels. Moreover, race differences in stimulated cortisol response in obese black and white adolescent females of the same sexual maturity have not been reported. In the present study, higher BMI was associated with lower stimulated increases in cortisol in response to ACTH in black girls. While others have shown serum cortisol levels and adrenocortical activity to be positively associated with fat mass in healthy children, our pilot data are in agreement with that of Dulin-Keita et al. (6), who reported a negative relationship between fat mass and serum cortisol levels. We hypothesize that this relationship is associated with increased conversion of cortisol to cortisone, regulated by 11 beta-hydroxysteroid dehydrogenase type 1, the activity of which is positively related to adiposity and likely genetically regulated (7).
Limitations of this pilot study are related to the small sample size and generalizability. Larger prospective studies of obese children will be necessary to confirm these initial results and to further differentiate racial determinants of adrenal hormone production in obesity. As these were children referred for treatment of obesity at an academic medical center, they are not necessarily representative of the pediatric population in the community.
In conclusion, there is accumulating data to indicate that race-related differences in adrenal response to ACTH stimulation may exist in obese adolescent females. The true effects of racial differences in adrenal hormone production in clinical medicine remain to be defined. This is deserving of further study, as measurements of androgen levels are commonly used in clinical practice and research for the assessment of conditions associated with obesity and insulin resistance.
Acknowledgments
This material is based on research supported by the U.S. Army Medical Research Acquisition Activity (820 Chandler Street, Fort Detrick MD 21702-5014), the awarding and administering acquisition office, Award Number W81XWH-06-2-0024. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Government. Other grant support included: U.S. Public Health Service Grant K24-HD01357 (SA), U.S. Public Health Service Grant K12-DK063704 Grant Scholar (TH; PI: SA), and the Pediatric Clinical and Translational Research Center at Children’s Hospital of Pittsburgh (NIH/NCRR/CTSA Grant UL1-RR024153).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002 Apr;87(4):1555–9. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 2.Lamon-Fava S, Barnett JB, Woods MN, McCormack C, McNamara JR, Schaefer EJ, et al. Differences in serum sex hormone and plasma lipid levels in Caucasian and African-American premenopausal women. J Clin Endocrinol Metab. 2005 Aug;90(8):4516–20. doi: 10.1210/jc.2004-1897. [DOI] [PubMed] [Google Scholar]
- 3.Randolph JF, Jr., Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003 Apr;88(4):1516–22. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 4.Richards RJ, Svec F, Bao W, Srinivasan SR, Berenson GS. Steroid hormones during puberty: racial (black-white) differences in androstenedione and estradiol--the Bogalusa Heart Study. J Clin Endocrinol Metab. 1992 Aug;75(2):624–31. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- 5.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005 Oct;90(10):5588–95. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 6.Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. J Epidemiol Community Health. 2010 Aug 24; doi: 10.1136/jech.2009.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsay RS, Wake DJ, Nair S, Bunt J, Livingstone DE, Permana PA, et al. Subcutaneous adipose 11 beta-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metab. 2003 Jun;88(6):2738–44. doi: 10.1210/jc.2002-030017. [DOI] [PubMed] [Google Scholar]