Abstract
Oropharyngeal candidiasis (OPC) is the most common oral infection in HIV+ persons. Previous studies suggest a role for CD8+ T-cells against OPC when CD4+ T-cells are lost, but enhanced susceptibility to infection occurs when CD8+ T-cell migration is inhibited by reduced tissue E-cadherin.
Objective
Conduct a longitudinal study of tissue CD8+ T-cells and E-cadherin expression before, during, and after episodes of OPC.
Methods
Oral fungal burden was monitored and tissue was evaluated for CD8+ T-cells and E-cadherin over a one-year period in HIV+ persons with a history of, or an acute episode of OPC.
Results
While longitudinal analyses precluded formal interpretations, point prevalence analyses of the dataset revealed that when patients experiencing OPC were successfully treated, tissue E-cadherin expression was similar to patients who had not experienced OPC, and higher numbers of CD8+ T-cells were distributed throughout OPC− tissue under normal expression of E-cadherin.
Conclusion
These results suggest that 1) reduction in tissue E-cadherin expression in OPC+ patients is not permanent, and 2) high numbers of CD8+ T-cells can be distributed throughout OPC− tissue under normal E-cadherin expression. Together these results extend our previous studies and continue to support a role for CD8+ T-cells in host defense against OPC.
Keywords: oropharyngeal candidiasis, HIV, CD8+ T-cells, E-cadherin
Introduction
Oropharyngeal candidiasis (OPC), caused by Candida albicans, is the most common oral infection in those with human immunodeficiency virus (HIV) infection (Greenspan et al., 1992; Klein et al., 1984; Laskaris et al., 1992; Macher, 1988). C. albicans is a ubiquitous opportunistic fungal organism that is part of the normal microflora of the gastrointestinal and reproductive tracts. As a result of early exposure, most healthy individuals exhibit Candida-specific immunity that protects against infection. However, under immunocompromised conditions, such as HIV infection, C. albicans’ opportunistic nature renders it capable of rapid conversion to a pathogen, causing symptomatic mucosal infections (Clift, 1984; Fichtenbaum and Powderly, 1998; Klein et al., 1984; Knight and Fletcher, 1971; Macher, 1988; Odds, 1988). Clinically, OPC can be observed in lesions as a mixture of hyphae and yeast, normally located in the stratum corneum-keratin layer of the outer epithelium, and can affect the buccal mucosa, gingival cuff, palate, and tongue. The infections can be erythematous, atrophic lesions that appear reddish, or pseudomembranous, white curd-like lesions, commonly referred to as thrush (Dodd et al., 1991). OPC can lead to difficulty in chewing, painful swallowing, and ultimately reduced nutritional consumption with significant morbidity (Fisher-Hoch and Hutwagner, 1995).
Cell-mediated immunity (CMI) by CD4+ Th1 and Th17-type cells is considered the predominant host defense mechanism against OPC (Conti et al., 2009; Greenspan et al., 1992; Leigh et al., 2001; Matsuzaki and Umemura, 2007; Reichart et al., 2000; Romani et al., 1991; Zelante et al., 2007; Zelante et al., 2009). This is consistent with the frequency of OPC in HIV+ persons when blood CD4+ T-cell numbers drop below 200 cells/μL. Despite the strong correlation of increased incidence of OPC in people with reduced blood CD4+ T-cells, immunological analyses have revealed little to no Candida-specific defects in blood CD4+ T-cells (Leigh et al., 2001). Thus, it is postulated that protection against OPC is multi-factorial, but primarily dependent on a threshold level of blood CD4+ T cells (usually 200 cells/uL). Below the CD4 cell threshold, systemic Th1/Th17-type CMI is no longer protective and protection becomes dependent on several local immune mechanisms, including Th cytokines in saliva, epithelial cell anti-Candida activity, and the local presence of CD8+ T-cells (Leigh et al., 1998; Myers et al., 2003; Steele et al., 2000).
Although CD8+ T-cells have not been considered prominent in host defense against Candida, in HIV+ persons, activated memory CD8+ T-cells have been shown to be recruited but accumulate at the lamina propria-epithelium interface within OPC lesions at a considerable distance from the site of infection at the outer epithelium (Myers et al., 2003). Hence, CD8+ T-cells are considered to play a role in host defense against OPC, but that a dysfunction in the cells or in the microenvironment precludes the migration of the cells through the mucosa for effector function.
T-cells express specific homing receptors that interact with cell adhesion molecules (CAMs) in tissue that facilitate the migration of cells (Hogg and Berlin, 1995). These interactions, initiated by binding of CAMs to reciprocal homing receptors play important roles in the mediation of the immune response against infections. In our HIV+ cohort a significant increase in epithelium-associated Mucosal addressin CAM (MadCAM) expression in OPC+ lesions was shown compared to OPC− tissues consistent with the recruitment of CD8+ T cells, whereas a significant reduction in E-cadherin expression was observed (McNulty et al., 2005). E-cadherin facilitates the migration of T cells through mucosal tissues. Therefore, we hypothesize that in OPC+ lesions, reduced E-cadherin is responsible for the lack of CD8+ T cell migration to the site of infection for effector function.
The purpose of this study was to conduct a longitudinal analysis to examine the extent of the reduced E-cadherin in OPC+ persons and further evaluate the presence of tissue-associated CD8+ T-cells during various times and conditions in the patients before, during, and after episodes of OPC.
Methods and Materials
Study Population
Patients were recruited and evaluated between May 2004 and October 2009 at Henry Ford Hospital in Detroit, as well as the Louisiana State University Health Sciences Center HIV+ Outpatient Dental Clinic affiliated with the HIV Outpatient Program (HOP) of the Medical Center of Louisiana at New Orleans and the Louisiana State University School of Dentistry. Informed consent was obtained from all patients, and all procedures were followed in the conduct of clinical research in accordance with the Institutional Review Boards at Henry Ford Hospital and Louisiana State University Health Sciences Center. The participating HIV+ subjects were part of a previously established cohort and contained 5 patients with no history of OPC (naïve) and 29 patients with a history of OPC. The cohort was 53% male and 79% African American with the remaining subjects classified as White/Caucasian. The subjects ranged from 33–53 years of age. A total of 22 patients who completed the study (17 with a history of OPC and 5 with no history of OPC) were used for this analysis. All of the patients enrolled in the study were on highly active antiretroviral therapy (HAART) and had <200 CD4+ T-cells/μl at the time of enrollment.
Diagnosis of Oropharyngeal candidiasis and detection of oral yeast colonization
The diagnosis of OPC and detection of oral yeast colonization was described previously (Myers et al., 2003; Slavinsky III et al., 2002). Briefly, diagnosis of OPC was made based on clinical appearance of oral mucosa, i.e. red, atrophic areas (erythematous) or while curd-like plaques (Dodd et al., 1991). Identification of OPC was further confirmed by hyphae or blastoconidia present on a wet mount slide preparation using potassium hydroxide (KOH), and a positive swab culture with characteristic colony morphology, as previously described (Myers et al., 2003), to confirm the presence of the organism. Only those patients with pseudomembranous OPC on the buccal mucosa were included in the cohort due to the site-specific nature of past studies (McNulty et al., 2005; Myers et al., 2003).
Specimen Collection/Processing
Swab
For longitudinal analysis, oral swabs were collected from the buccal mucosa, palate, tongue, and whole mouth every 2 weeks. Each swab was cultured on Sabouraud Dextrose Agar (Becton Dickinson Microbiology Systems, Franklin, NJ) and Chromagar (CHROMagar Microbiolgy, Paris, France). Initial speciation was screened for by color on Chromagar. Colonies of each color were identified to species level by API biochemical tests (API ID 32C; BioMerieux, Durham, NC). Of the enrolled patients completing the study, 93% were colonized at baseline. C. albicans was detected in all those colonized. On average, these colonized patients remained colonized throughout the study (~90% of subsequent visits). Of those not colonized at baseline, several remained uncolonized (~54% of subsequent visits). In addition to C. albicans, 43% of patients were also colonized with C. glabrata (n=9), 18% with C. tropicalis (n=4), 9% with C. dubliniensis (n=2), and 9% with C. krusei (n=2).
Saliva
Unstimulated saliva (10ml) from each subject was expectorated into a polypropylene test tube before each biopsy was collected. A portion of each sample (50 μl) was cultured and speciated as described above. The remaining saliva was clarified by centrifugations, sterile filtered, and stored at −80°C in 1ml aliquots for future analysis.
Biopsy
Buccal mucosa biopsies were collected as described previously (Myers et al., 2003). For the present study, biopsies were collected every 2 months or upon an acute episode of OPC (not less than 3 weeks from a prior scheduled biopsy). The biopsied area was also swabbed before the biopsy was performed and any colonies on the plates speciated as above. Tissue was embedded in Tissue-Tek Optimal Cutting Temperature (OCT) medium contained in a Tissue-Tek Cryomold (Sakura Finetek; Torrance, CA) and frozen over dry ice. Specimens were stored at −80°C until sectioned. For immunohistochemistry, tissue sections (5 μm) were collected on glass slides, fixed in ice-cold acetone (5 min), and stored at −20°C.
Immunohistochemistry
Immunohistochemical staining of buccal mucosa was performed as previously described (McNulty et al.. 2005; Myers et al., 2003). Briefly, all steps were performed using Anti-Mouse Cell and Tissue Staining Kit (horseradish peroxidase-3-amino-9-ethylcarbazole; R&D Systems, Minneapolis, MN). Serial sections were rehydrated in phosphate-buffered saline (PBS); blocked with 3% hydrogen peroxide, mouse serum, avidin, and biotin; and then incubated overnight at 4°C with mouse anti-human CD8 (1μg; Serotec, Raleigh, NC), mouse anti-human E-cadherin (1μg; Dako, Carpinteria, CA), or isotype control mouse IgG1 (1 μg; Serotec). The slides were then washed and incubated with appropriate anti-mouse (R&D Systems) biotinylated IgG secondary antibody (5μg/mL) for 1 h. The washed slides were then incubated for 30 minutes with high sensitivity streptavidin-horseradish peroxidase conjugate (R&D Systems), washed, and incubated with the substrate 3-amino-9-ethylcarbazole chromagen (R&D Systems) for 10 min. CAT hematoxylin (Biocare Medical, Concord, CA) was used to counterstain. The slides were preserved by using aqueous mounting medium (R&D Systems). CD8+ T-cells and E-cadherin in the lamina propria and epithelium were scored visually and assigned a score of + to ++++ based on numbers (CD8 cells) or intensity (Lin et al. 2004).
Morphometric Analysis
Analysis was performed as described previously (McNulty et al., 2005). Briefly, using bright-field microscopy, 10–12 adjacent areas at 4x or 2 to 4 adjacent areas at 10x (~37,000 μm2/each) per slide were identified in areas of cell-concentration (usually the lamina propria-epithelial border). These areas were gated, and positively stained T-cells or stained areas were marked (“painted”) using MetaView software (Universal Imaging Corp, Downington, PA). A percent threshold of positively stained cells per multiple units of area was quantified for each patient tested. These values were used to determine the mean percent threshold and standard error of the mean for each patient group.
Statistics
For longitudinal analyses, repeated measures ANOVA and other non-parametric tests (Mann-Whitney, Wilcoxon, ANOVA) were employed. For point prevalence analyses, the Student’s t test was employed. Significant differences were defined as p<0.05 using two-tailed test. Statistics were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Classification of the cohort
In order to evaluate the presence of tissue-associated CD8+ T-cells and E-cadherin expression, we first classified the study population according to fungal burden and the incidence of OPC. The cohort was divided into three subsets. One was patients that acquired an episode of OPC over the course of the study (OPC+ patients, n=6). For these patients, the baseline CD4+ T-cells/μl and viral load (copies/ml) were 165 and 192,090, respectively. Over the course of the study, the average CD4 counts were 238 cells/μl and average viral load was 74,883 copies/ml. A second were OPC− patients classified as ‘unremarkable’ with low, asymptomatic oral fungal burden throughout the course of the study (n=10). For these patients, the baseline CD4+ T-cells/μl and viral load (copies/ml) were 160 and 156,729, respectively. Over the course of the study, the average CD4 counts were 409 cells/μl and average viral load was 78,497 copies/ml. The third were patients who had a putative ‘preclinical’ condition, exhibiting high, asymptomatic fungal burden at varying timepoints during the study, but never developed OPC (n=6). For these patients, the baseline CD4+ T-cells/μl and viral load (copies/ml) were 131 and 914,617, respectively. Over the course of the study the average CD4 counts were 163 cells/μl and average viral load was 460,368 copies/ml.
Fungal burden, tissue-associated CD8+ T cells, and E cadherin expression
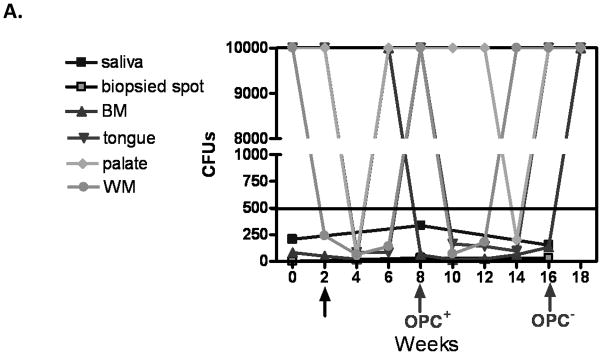
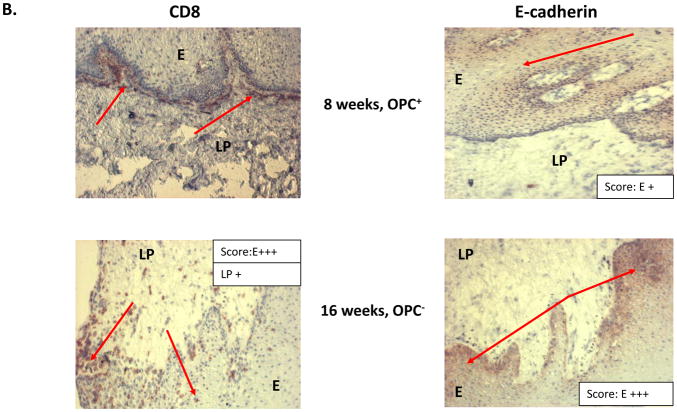
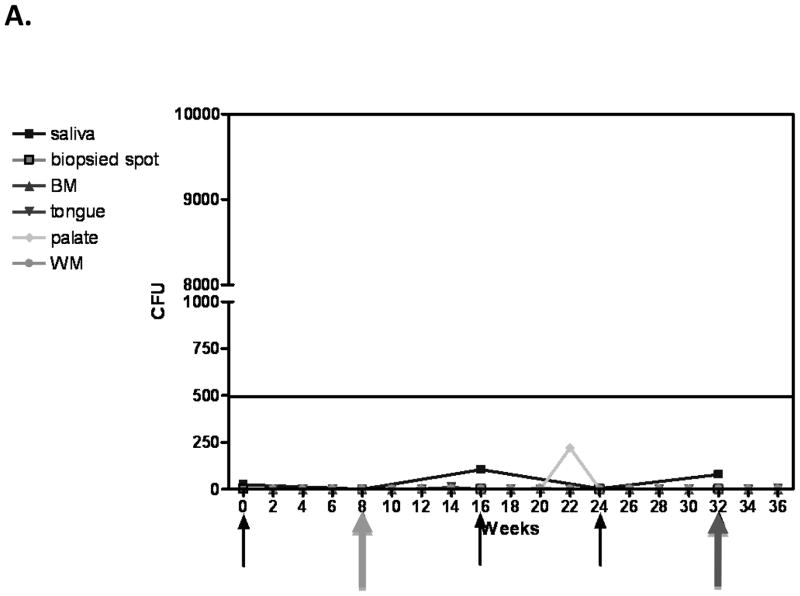
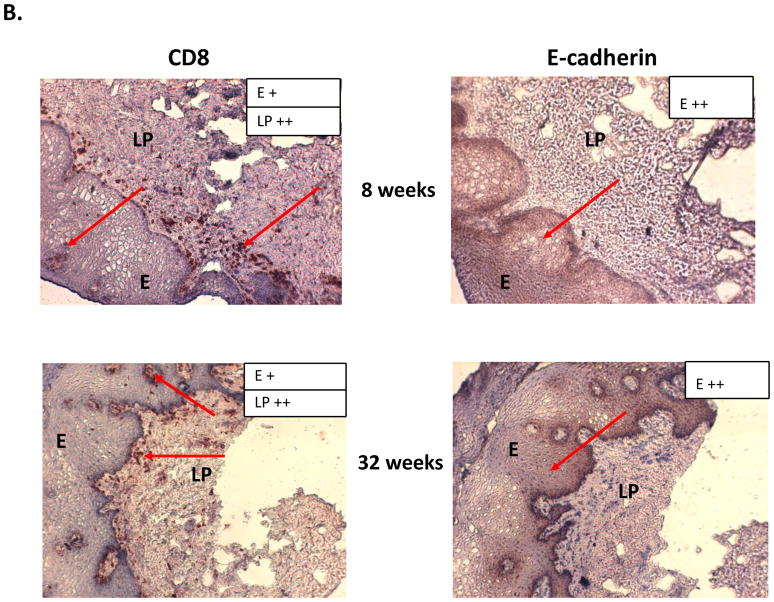
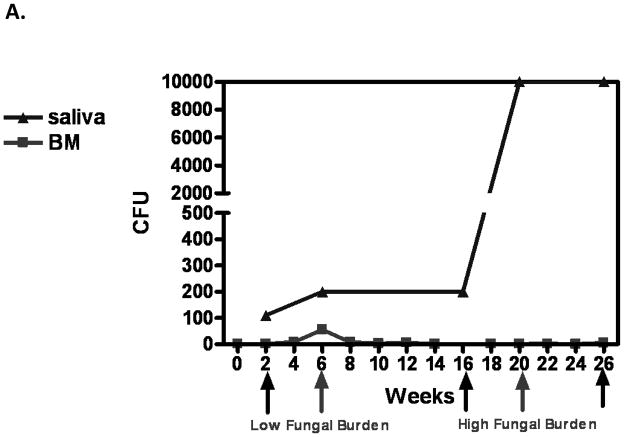
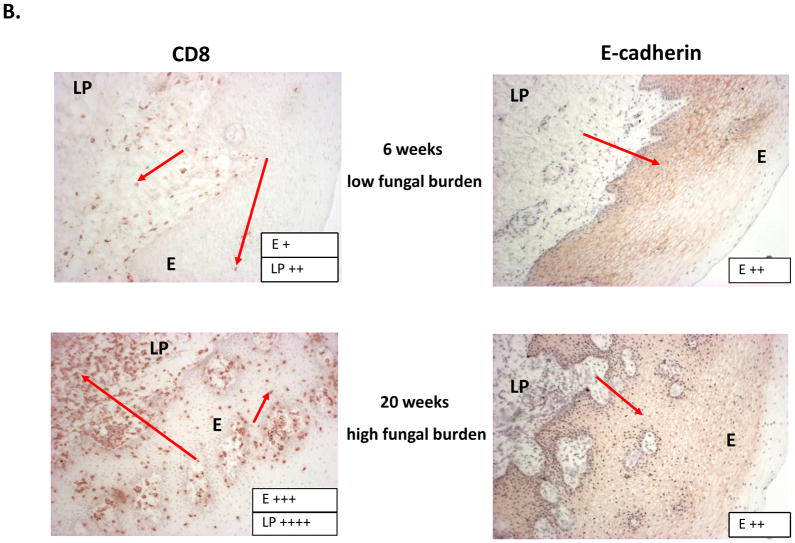
Results of the longitudinal study were highly variable relative to fungal burden, and tissue-associated CD8+ T-cells and E-cadherin expression. Yet there were some representative/predicted phenotypes in each subset. For patients that had one or more episodes of OPC during the study (OPC+ patients), oral fungal burden was variable at any given time. Biopsies stained for CD8+ T-cells and E-cadherin during episodes of OPC showed the hallmark accumulation of CD8+ T-cells at the epithelium-lamina propria interface coupled with reduced E-cadherin expression. At times post-treatment and an OPC− status, CD8+ T-cells were often observed throughout the tissue in varying numbers with E-cadherin expression generally increased. Data for a representative patient of this predicted phenotype during (8 weeks) and after (16 weeks) an episode of OPC is shown in Figure 1 (A- fungal burden, B- CD8 and E-cadherin+ staining). Scores reflect differences in relative expression. In patients with low oral fungal burden throughout the course of the study, tissue-associated CD8+ T-cells were observed in small numbers with normal tissue E-cadherin expression. Data from a representative patient of this predicted phenotype of two time points is shown in Figure 2 (A- fungal burden, B- CD8 and E-cadherin+ staining). In patients classified as pre-clinical, swab and saliva cultures revealed times of both low and high oral fungal burden over the course of the study. During periods of low fungal burden, tissue-associated CD8+ T-cells and E-cadherin were similar to the unremarkable subset. During periods of high, asymptomatic fungal burden (putative pre-clinical condition), highly variable numbers of CD8+ T-cells were observed often throughout the tissue, with E-cadherin expression predominately in a range considered normal. Data from a representative patient with this predicted phenotype at low (6 weeks) and high (20 weeks) fungal burden is shown in Figure 3 (A- fungal burden, B- CD8 and E-cadherin+ staining). Scoring again reflects changes in relative expression.
Figure 1.
Representative patient with an acute episode of OPC pre- and post- antifungal treatment. (A) Evaluation of fungal burden over time. Arrows indicate when biopsies were taken over the course of study. CFU=colony forming units, BM=buccal mucosa, WM=whole mouth. (B) Immunohistochemical evaluation of CD8+ T cells and E-cadherin expression pre- and post-treatment with antifungal therapy. Magnification 10x. Red arrows point to positive staining. E=epithelium, LP=lamina propria. Scores for relative expression (+ to ++++) are provided for LP and/or E.
Figure 2.
Representative patient classified as ‘unremarkable’ phenotype. (A) Evaluation of fungal burden over time. Arrows indicate when biopsies were taken over the course of study. CFU=colony forming units, BM=buccal mucosa, WM=whole mouth. (B) Immunohistochemical evaluation of CD8+ T cells and E-cadherin expression over time. Magnification 10x. Red arrows point to positive staining. Scores for relative expression (+ to ++++) are provided for LP and/or E.
Figure 3.
Representative patient exhibiting an OPC− preclinical condition. (A) Evaluation of fungal burden over time. Arrows indicate when biopsies were taken over the course of study. CFU=colony forming units, BM=buccal mucosa. (B) Immunohistochemical evaluation of CD8+ T cells and E-cadherin expression during times of low and high fungal burden. Magnification 10x. Red arrows point to positive staining. Scores for relative expression (+ to ++++) are provided for LP and/or E.
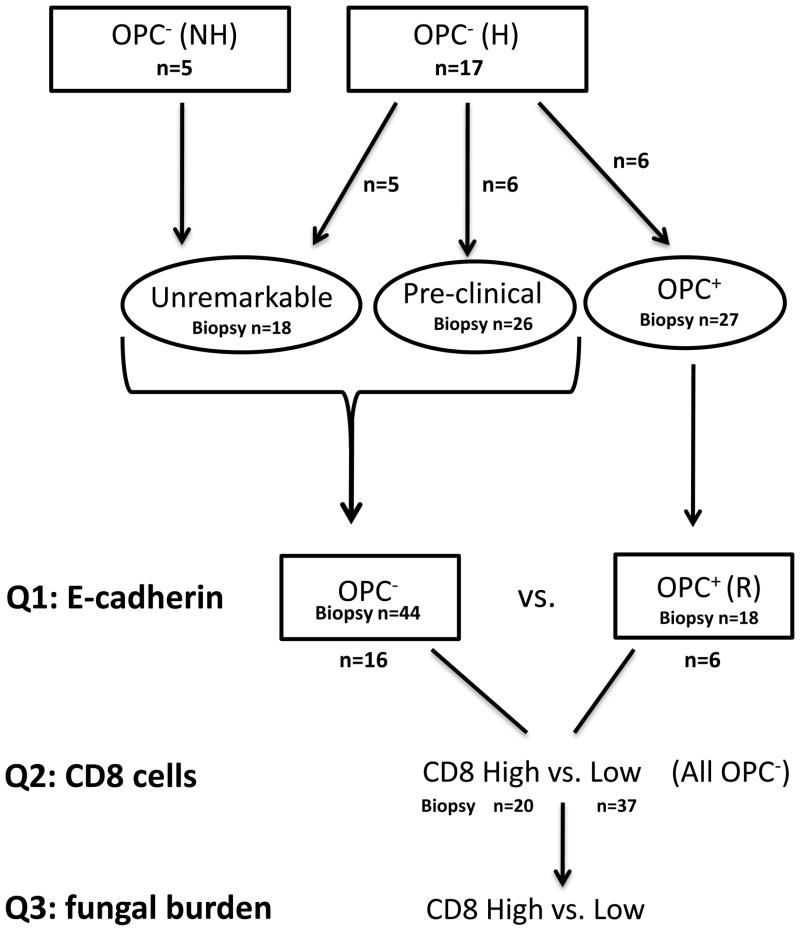
Due to the highly variable nature of these data, morphometric analyses on a per patient basis or as a group of patients over time did not allow for any reasonable interpretations, statistically, regarding correlates between CD8 T-cell presence, E-cadherin expression, and oral fungal burden despite the representative phenotypes observed. Instead, point prevalence analyses were conducted to address three specific questions. Figure 4 shows a flowchart of how the original patient groups were stratified and how the number of total samples (biopsies) were stratified for the point prevalence analyses and used to address three primary questions.
Figure 4.
Classification of the cohort. Patients at enrollment who had a history (H) or no history (NH) of OPC were stratified into 3 groups according to fungal burden and the incidence of OPC during the study (Unremarkable, Pre-clinical, and OPC+). Further stratification of patients [continued OPC− or treated and OPC− in remission (R)], and tissues (high or low CD8+ T cells) was done to address the 3 primary questions (Q) of the point prevalence analyses.
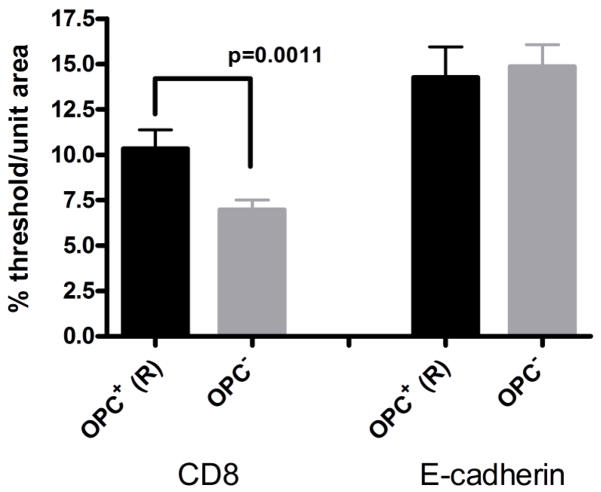
The first question was: In patients that developed OPC, is the reduced E-cadherin expression permanent or transient? To determine if E-cadherin degradation is permanent in those with OPC, an analysis was performed comparing E-cadherin expression in those patients who never developed OPC during the course of the study (unremarkable and preclinical subsets) (patients- n=11; samples- n=44) and patients who had developed OPC during the course of the study tested during times of remission after successful antifungal treatment (OPC− condition) (patients- n=6; samples- n=18). Results in Figure 5 show there was no difference in E-cadherin expression between these two data sets suggesting that the reduced E-cadherin during OPC is a transient condition. There was also a significant increase in tissue-associated CD8+ T-cells during remission in those that had developed OPC.
Figure 5.
CD8+ T cells and E-cadherin expression in oral mucosal biopsies of OPC+ patients in times of remission (R) (post antifungal therapy) compared to OPC− patients. For morphometric analysis, a percent threshold of positive staining was quantified per multiple units (n=10–12) of area (~37,000 μm2/each) and averaged for each biopsy tested. OPC+ (R): n=6 patients, 18 samples; OPC−: n=11 patients, 44 samples. Significant differences are denoted (CD8 cells in tissue from OPC+ patients now OPC− in remission compared to patients who were OPC−throughout the study - P = 0.0011).
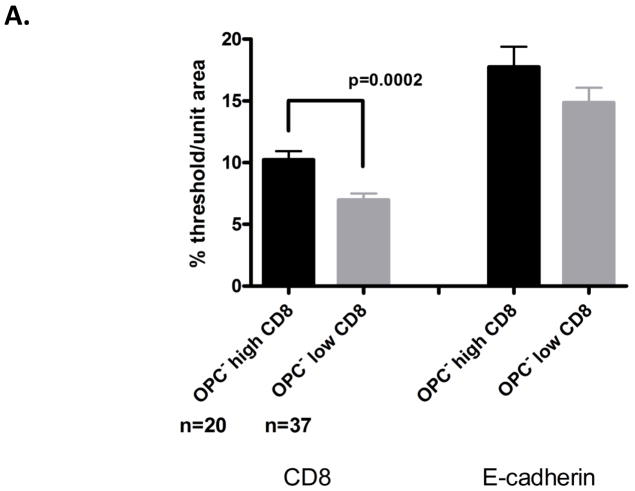
The second question was: Do CD8+ T-cells increase in OPC− tissue under normal E-cadherin expression? To determine if CD8+ T-cells were ever increased in OPC− tissue expressing normal levels of E-cadherin (~15–20% threshold/unit area for OPC− patients), an analysis was conducted after arbitrarily assigning OPC− biopsy tissues as ‘high’ and ‘low’ relative to tissue CD8+ T-cells numbers (Figure 4). These tissues were assigned based on reported baseline levels in normal, healthy tissues (~5–7% threshold/unit area) (Myers et al., 2003). The assignments resulted in 20 tissues categorized as ‘high’ CD8+ T-cells (~9–16% threshold/unit area) and 37 tissues categorized as ‘low’ CD8+ T-cells. These tissues were taken from a total of 22 patients, including OPC+ patients in remission. Morphometric comparisons of biopsies assigned to these two groups revealed a statistically significant increase in tissue CD8+ T-cells for the high CD8+ T-cell group. On further examination of these groups, the increased CD8+ T-cells occurred in 64% of patients without OPC and in 56% of biopsies taken from these patients (Figure 6A). In considering the distribution of the CD8+ T-cells in the tissues of the high CD8 cell group, cells were observed to be widely distributed throughout the lamina propria and epithelium. Tissue E-cadherin expression was confirmed as similar in both groups.
Figure 6.
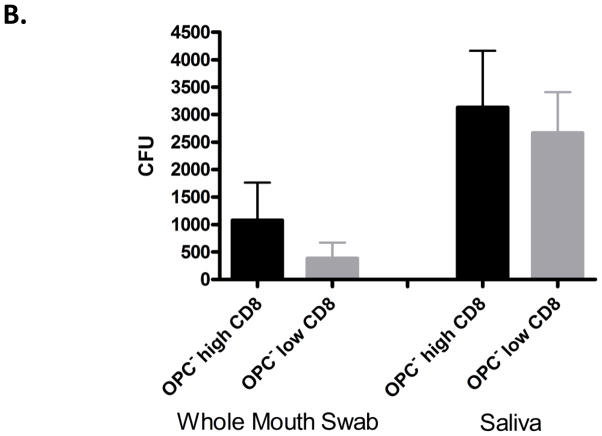
CD8+ T cell distribution under ‘normal’ E-cadherin expression (A) Morphometric analysis of CD8+ T cells and E-cadherin in oral mucosal biopsies of OPC− patients. The analysis was conducted after arbitrarily assigning OPC− biopsies as ‘high’ and ‘low’ relative to tissue CD8+ T cell numbers. These tissues were taken from a total of 22 patients, including OPC+ patients in remission. A percent threshold of positive staining was quantified per multiple units of area (n=10–12) (~37,000 μm2/each) and averaged for each biopsy tested. Significant differences are denoted (OPC− tissue with high CD8 T cells compared to OPC− tissue with low CD8 T cells - P=0.002). (B) Evaluation of fungal burden during times of ‘increased’ CD8+ T cells compared to tissues with low CD8+ T cell numbers. CFU=colony forming units.
The third question was: Is oral fungal burden increased concomitantly with increased CD8+ T-cells? Oral Candida colonization from whole mouth swab and saliva was not significantly increased in those patients with high tissue-associated CD8+ T-cells compared to those with low CD8+ T-cells, although a trend toward higher levels of colonization was observed (Figure 6B).
Discussion
CD8+ T-cells are hypothesized to play a role in the host defense against OPC as evidenced by their presence in large numbers and an activated state along the epithelial-lamina propria interface in OPC+ lesions (Myers et al., 2003). Yet the considerable distance from the site of Candida infection at the outer epithelium suggests a potential dysfunction in the tissue microenvironment, namely reduction in the mucosal cell trafficking molecule E-cadherin, for effective migration of the CD8+ T-cells and adequate effector function against Candida.
To better understand the clinical observations and test the hypothesis, a longitudinal study was conducted. One important issue was to determine if reduced E-cadherin expression during times of OPC was a permanent or transient condition. Equally critical was that if CD8+ T-cells do play a role in resistance to or protect against OPC, are there times when their levels increase and are widely distributed throughout the tissue with potential for effector function rather than being essentially “stuck” at the epithelial-lamina propria interface?
The longitudinal study consisted of those with and without a history of OPC. Furthermore, we classified the patients into three subsets based on fungal burden and occurrence of OPC. The ‘unremarkable’ subset was patients with or without a history of OPC with low asymptomatic oral fungal burden throughout the course of the study. The ‘preclinical’ subset was those patients with or without a history of OPC having high, asymptomatic oral fungal burden at various times, but who never developed OPC during the study. The ‘OPC+’ subset was those who developed OPC during the course of the study. While these subsets showed some representative snapshot phenotypes suggesting that the reduced E-cadherin in OPC+ patients was not permanent and CD8+ T cells could migrate throughout the tissue in higher numbers in OPC−tissue (Figures 1–3), unfortunately these data analyzed per patient over time for these subsets were highly dynamic. Hence, formal interpretations could not be made regarding associations between the presence of CD8+ T-cells, E-cadherin expression, and oral fungal burden between the subsets.
Instead, point prevalence analyses were conducted using the entire dataset from the longitudinal study and asking three primary questions. First, was whether E-cadherin reduction in OPC+ patients is a permanent or transient condition? Results clearly showed that tissue E-cadherin in OPC+ patients during times of remission (after successful antifungal treatment) was similar to that for other OPC− patients, suggesting a transient reduction. This transient reduction in E-cadherin provides the potential for CD8+ T-cell migration at certain times in those susceptible to OPC, including those with a significant history of episodes. Indeed, because CD8+ T-cells were increased in these OPC+ patients during times of remission (having had the hallmark accumulation of CD8+ T-cells when OPC+) these data provide additional evidence for the potential that recruited CD8+ T-cells can migrate and be engaged for effector function in OPC patients.
Second, do CD8+ T-cells increase in OPC− tissues under normal expression of E-cadherin? Results indeed showed increased CD8+ T-cells in at least one biopsy from 64% of OPC− patients. In fact, this distribution occurred on average in 56% of the biopsies from such patients. Furthermore, evaluation of the tissue sections showed a wide distribution of the cells throughout the lamina propria and epithelium. While these results suggest that the CD8+ T-cells can migrate widely in the tissue in higher numbers, to analyze the levels of CD8+ T-cells using a point prevalence analysis, biopsies were arbitrarily declared as being either high or low relative to tissue CD8+ T-cell numbers. Although arbitrary, a good distribution was obtained with reasonable sample size and data showed a statistical difference between the two groups (p=0.002). Hence, we feel these data are representative.
Third, is oral Candida colonization increased during times of increased CD8+ T-cells? Results showed no statistical increase in fungal burden in tissues with higher CD8+ T-cells although a trend toward an increase was observed. We recognize that additional time points and data from more patients may show larger differences in fungal burden.
Overall, the results from the point prevalence analyses confirmed the broad, yet distinct snapshot phenotypes from the longitudinal data and support our hypothesis that 1) the reduction in E-cadherin expression is not permanent in OPC+ cases, and 2) that CD8+ T-cells can be present with considerable distribution throughout the tissue in OPC− patients under normal E-cadherin expression. This provides yet more evidence for the role of CD8+ T-cells in host defense against OPC when CD4+ T-cells are reduced. The results also suggest the potential to enhance CD8+ T-cell migration and function via immunotherapies. Agents that increase E-cadherin would predict more efficient CD8 T-cell mobilization and effector function. Interestingly, recent data show that Candida itself can degrade E-cadherin through their secretory aspartyl proteases (SAPs) (Frank and Hostetter, 2007; Schaller et al., 1999; Villar et al., 2007). This represents a possible means by which E-cadherin is reduced followed by subsequent immobilization of CD8+ T-cells to the site of infection. It may also represent a virulence property of Candida for stronger tissue adherence and invasion. If one considers the dramatic reduction in OPC during the post-antiretorviral therapy (ART) era (Calderone and Fonzi, 2001; Cassone et al., 1999; Gruber et al., 1999; Palella, Jr. et al., 1998), it is interesting to postulate that in addition to the immune reconstitution of CD4+ T-cells, ART may be effective in reversing the degradation of E-cadherin and in turn allow effective CD8+ T-cell migration and anti-Candida function. Support for this comes from the direct anti-Candida activity of protease inhibitors (PI), often included in ART, that are known to inhibit Candida SAPs (Borg-von Zepelin et al., 1999; Cassone et al., 1999; Korting et al., 1999). Hence, ART may be considered immunotherapeutic in multiple ways. Current studies are focused on testing this directly as well as dissecting the function of the tissue-associated CD8+ T-cells against Candida.
Acknowledgments
This work was supported by National Institute of Health Public Health Service grants DE 12178 (Fidel) from the National Institute of Dental and Craniofacial Research.
This work was also supported in part by the Louisiana Vaccine Center and South Louisiana Institute for Infectious Diseases Research sponsored by the Louisiana Board of Regents.
References
- 1.Borg-von Zepelin M, Meyer I, Thomssen R, Würzner R, Sanglard D, Telenti A, Monod M. HIV-Protease inhibitors reduce cell adherence of Candida albicans strains by inhibition of yeast secreted aspartic proteases. J Invest Dermatol. 1999;113:747–751. doi: 10.1046/j.1523-1747.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- 2.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 3.Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180:448–453. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 4.Clift RA. Candidiasis in the transplant patient. Amer J Med. 1984;77(suppl 4D):34–38. [PubMed] [Google Scholar]
- 5.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd CL, Greenspan D, Katz MH, Westenhouse JL, Feigal DW, Greenspan JS. Oral candidiasis in HIV infection: pseudomembranous and erythematous candidiasis show similar rates of progression to AIDS. AIDS. 1991;5:1339–1343. [PubMed] [Google Scholar]
- 7.Fichtenbaum CJ, Powderly W. Refratory mucosal Candidiasis in patients with human immunodeficiency virus infection. Clin Infect Dis. 1998;26:556–565. doi: 10.1086/514571. [DOI] [PubMed] [Google Scholar]
- 8.Fisher-Hoch SP, Hutwagner L. Opportunistic candidiasis: An epidemic of the 1980s. Clin Infect Dis. 1995;21:897–904. doi: 10.1093/clinids/21.4.897. [DOI] [PubMed] [Google Scholar]
- 9.Frank CF, Hostetter MK. Cleavage of E-cadherin: a mechanism for disruption of the intestinal epithelial barrier by Candida albicans. Transl Res. 2007;149:211–222. doi: 10.1016/j.trsl.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan JS, Barr CE, Sciubba JJ, Winkler JR. Oral manifestations of HIV infection: definitions, diagnostic criteria and principles of therapy. Oral Surg Oral Med Oral Pathol. 1992;73:142–144. doi: 10.1016/0030-4220(92)90185-s. [DOI] [PubMed] [Google Scholar]
- 11.Gruber A, Speth C, Lukasser-Vogl E, Zangerle R, Borg-von Zepelin M, Dierich MP, Wurzner R. Human immunodeficiency virus type 1 protease inhibitor attenuates Candida albicans virulence properties in vitro. Immunopharmacol. 1999;41:227–234. doi: 10.1016/s0162-3109(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 12.Hogg N, Berlin C. Structure and function of adhesion receptors in leukocyte trafficking. Immunol Today. 1995;16:327–330. doi: 10.1016/0167-5699(95)80147-2. [DOI] [PubMed] [Google Scholar]
- 13.Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–357. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 14.Knight L, Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteriods and diabetes mellitus. J Infect Dis. 1971;123:371–377. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- 15.Korting HC, Schaller M, Eder G, Hamm G, Böhmer U, Hube B. Effects of human immunodeficiency virus (HIV) proteinase inhibitors saquinavir and indinavir on in vitro activities of secreted aspartyl proteinases of Candida albicans isolates from HIV-infected patients. Antimicrob Agents Chemother. 1999;43:2038–2042. doi: 10.1128/aac.43.8.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laskaris G, Hadjivassiliou M, Stratigos J. Oral signs and symptoms in 160 Greek HIV-infected patients. J Oral Pathol. 1992;21:120–123. doi: 10.1111/j.1600-0714.1992.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 17.Leigh JE, Barousse M, Swoboda RK, Myers T, Hager S, Wolf NA, Cutright JL, Thompson J, Sobel JD, Fidel PL., Jr Candida-specific systemic cell-mediated immune reactivities in HIV-infected persons with and without mucosal candidiaisis. J Infect Dis. 2001;183:277–285. doi: 10.1086/317944. [DOI] [PubMed] [Google Scholar]
- 18.Leigh JE, Steele C, Wormley FL, Jr, Luo W, Clark RA, Gallaher W, Fidel PL., Jr Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: A pilot study in HIV-positive individuals with oropharyngeal candidiasis. J Acq Immun Defic Syndr Human Retrovirol. 1998;19:373–380. doi: 10.1097/00042560-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Macher AM. The pathology of AIDS. Public Health Report. 1988;103:246–254. [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 21.McNulty KM, Plianrungsi J, Leigh JE, Mercante DE, Fidel PL. Characterization of the CD8+ T-cells and microenvironment in oral lesions of HIV-infected persons with oropharyngeal candidiasis. Infect Immun. 2005;73:3659–3667. doi: 10.1128/IAI.73.6.3659-3667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers TA, Leigh JE, Arribas A, Hager S, Clark RA, Lilly E, Fidel PL., Jr Immunohistochemical evaluation of T cells in oral lesions from human immunodeficiency virus-positive persons with oropharyngeal candidiasis. Infect Immun. 2003;71:956–963. doi: 10.1128/IAI.71.2.956-963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odds FC. Candida and candidosis. Baltimore, MD: University Park Press; 1988. Chronic mucocutaneous candidiosis; pp. 104–110. [Google Scholar]
- 24.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Eng J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 25.Reichart PA, Samaranayake LP, Philipsen HP. Pathology and clinical correlates in oral candidiasis and its variants: a review. Oral Dis. 2000;6:85–91. doi: 10.1111/j.1601-0825.2000.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 26.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: Association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaller M, Korting HC, Schäfer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol. 1999;34:169–180. doi: 10.1046/j.1365-2958.1999.01590.x. [DOI] [PubMed] [Google Scholar]
- 28.Slavinsky J, III, Myers T, Swoboda RK, Leigh JE, Hager S, Fidel PL., Jr Th1/Th2 cytokine profiles in saliva of HIV-positive smokers with oropharyngeal candidiasis. Oral Microbiol Immunol. 2002;17:38–43. doi: 10.1046/j.0902-0055.2001.00080.x. [DOI] [PubMed] [Google Scholar]
- 29.Steele C, Leigh JE, Swoboda RK, Fidel PL., Jr Growth inhibition of Candida by human oral epithelial cells. J Infect Dis. 2000;182:1479–1485. doi: 10.1086/315872. [DOI] [PubMed] [Google Scholar]
- 30.Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and Protease Sap5p. Infect Immun. 2007;75:2126–2135. doi: 10.1128/IAI.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna M, Vacca C, Conte C, Mosci P, Bistoni F, Puccette P, Kastelein R, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2680–2682. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 32.Zelante T, De Luca A, D'Angelo C, Moretti S, Romani L. IL-17/Th17 in antifunal immunity: what's new? Eur J Immunol. 2009;39:645–648. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]