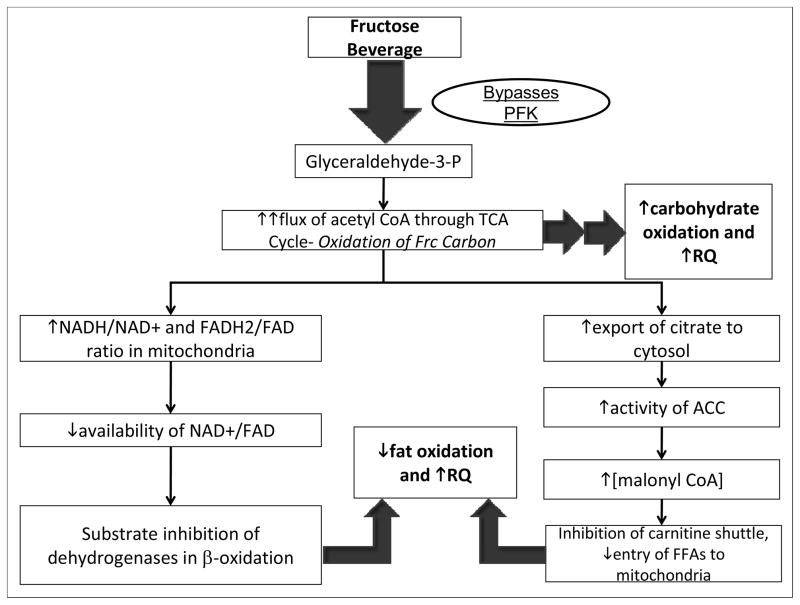
Figure 3.
Proposed mechanisms contributing to observed changes of substrate utilization in subjects consuming fructose-sweetened beverages. In the liver fructose is phosphorylated by fructokinase (which is not regulated by cellular energy status) and largely bypasses phosphofructokinase (PFK), the enzyme catalyzing the rate-limiting step of glycolysis (which is subject to inhibition by ATP and citrate). Ultimately fructose enters the glycolytic pathway as glyceraldehyde-3-phosphate. Following a high-fructose meal, an unregulated flux of fructose (Frc) carbon upregulates carbohydrate metabolism in the liver (increased CHO-Ox), leading to an increased flux of acetyl CoA through the tricarboxylic acid (TCA) cycle and a concomitant increase in cellular energy status (increased ATP/ADP ratio and NADH/NAD+ ratio). A high NADH/NAD+ ratio in the mitochondria results in substrate inhibition of isocitrate dehydrogenase (ICD) in the TCA cycle, leading to increased export of citrate to the cytosol, activation of acetyl-CoA carboxylase (ACC), and increased production of malonyl-CoA, the precursor to fatty acid synthesis (DNL). Elevated cytosolic concentrations of malonyl-CoA inhibit the carnitine shuttle via carnitine palmitoyl transferase (CPT), leading to reduced entry of fatty acids (FAs) into the mitochondria, decreased fat oxidation. The elevation of cellular energy status following a high-fructose meal would also lead to reduced mitochondrial availability of the fixed pool of oxidized cofactors NAD+ and FAD, which are required substrates for β-oxidation, also resulting in reduced fat oxidation (Locke et al 2008, Mayes 1993, McGarry 1995, Williamson and Cooper 1980).