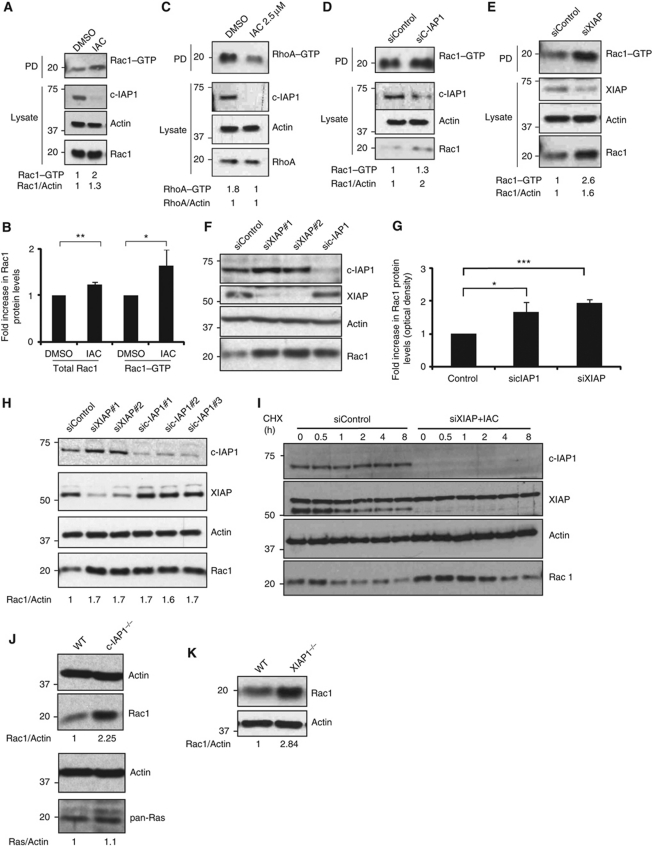
Figure 2.
IAPs regulate the stability of Rac1. (A) HeLa cells were treated either with IAC (2.5 μM) or with DMSO for 16 h and the active GTP-bound form of active Rac1 or RhoA (C) was precipitated as mentioned in Materials and methods. (B) Quantification of total and active Rac1 levels (by densitometry as described in Materials and methods) in control and IAC-treated cells is shown. Data from three independent experiments are shown. Error bars represent ±s.d. of the mean. The depletion of c-IAP1 and the total levels of Rac1 and RhoA were monitored in the total lysates. (D) HeLa cells were transfected with c-IAP1 or (E) XIAP siRNAs for 48 h and the amount of GTP-bound Rac1 was precipitated. The levels of total Rac1 were monitored in the cell lysates by immunoblots. (F) HeLa cells were transfected with various siRNAs for 48 h. Total cell lysates were western blotted for monitoring the protein levels of various IAPs and Rac1. (G) The increase in Rac1 levels was quantified by densitometry. The data from three independent experiments are shown (*P<0.05, **P<0.01, ***P<0.005). Error bars represent ±s.d. of the mean. (H) 293T cells were transfected with two sets of XIAP and three sets of c-IAP1 siRNAs. Rac1 levels were monitored by western blots. Quantification of Rac1 increase is provided. (I) Combined loss of XIAP and c-IAP1 enhances the protein half-life of Rac1. HeLa cells transfected with XIAP siRNAs were treated with IAC and cycloheximide was added to cells to monitor the protein half-life of Rac1. (J) Western blot analyses showing protein levels of Rac1 and Ras in wild-type (WT) and c-IAP1−/− or (K) WT and XIAP−/− MEFs. The signalling intensities of Rac1 and Ras bands were quantified by densitometry and normalized to actin levels. Figure source data can be found in Supplementary data.