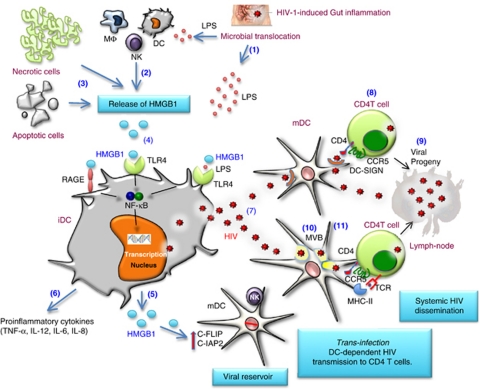
Figure 6.
Proposed contribution of HMGB1 to HIV persistence and dissemination. HIV disease progression is characterized by gut inflammation and consequently microbial translocation leading to the release of LPS in the bloodstream (1). Circulating LPS may induce active release of HMGB1 by innate cells, including macrophages and DCs (2). Necrotic and apoptotic cells that accumulate during chronic HIV infection may also be a constant source of HMGB1 (3). HMGB1 activates DCs by signaling through the receptor RAGE or TLR4 if cooperate with TLR4 ligand LPS, thus triggering NF-κB activation (4). This results in the release of HMGB1 (5) and proinflammatory cytokines (6), and the triggering of HIV replication in mDCs (7). Trans-infection of HIV from mDCs to CD4 T cells involves HIV capture by DC-SIGN followed by its recruitment at the site of T-cell interaction (8). This infectious synapse will lead to the productive infection of CD4 T cells (9). Trans-infection of HIV can also be mediated by exocytosis of the HIV-1 particles captured by DCs. After endocytosis, the captured HIV-1 particles are targeted to a multi-vesicular endosomal body (MVB) in DCs (10). Although some of the MVB-localized virus fraction is targeted to the lysosome and degraded to be further presented to TCR in the context of MHC molecules, fusion of MVB with the plasma membrane results in the release of virus particles along with exosomes (11). Virus produced by infected CD4 T cells, DCs and macrophages spread the infection to the draining lymph nodes and other lymphoid tissue