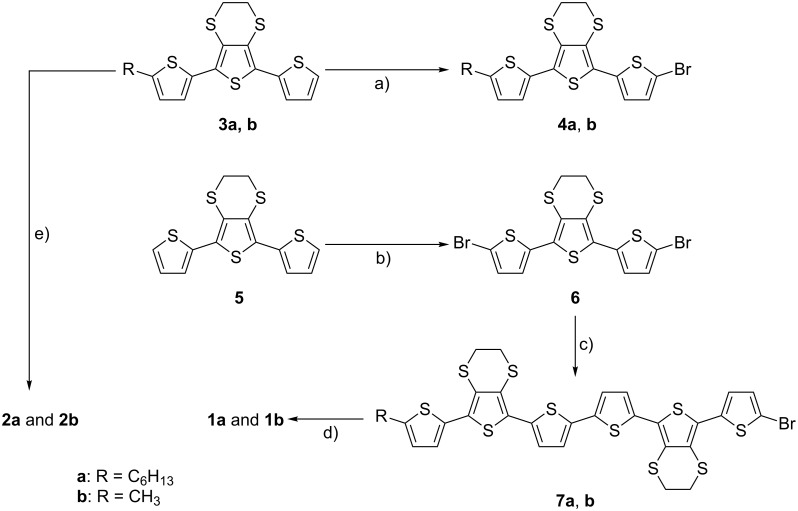
Scheme 1.
The synthesis of functionalised oligothiophenes 1a,b and 2a,b. Reagents and conditions: a) NBS, CH3CO2H, THF (1:1, v/v); 4a, 97%, 4b, 88%; b) 2.2 equiv NBS, CH3CO2H, THF (1:1, v/v); 6, 91%; c) (i) n-BuLi, (ii) ZnCl2, (iii) 4a or 4b, Pd(PPh3)4, THF; 7a, 40%, 7b, 20%; d) 4-(dimethylamino)phenylboronic acid, Pd(PPh3)4, toluene, EtOH, NaHCO3, H2O; 1a, 28%, 1b, 44%; e) FeCl3, CHCl3; 2a, 40%, 2b, 50%.