Table 1.
18O-labeling study of amide synthesis from α-bromo nitroalkanes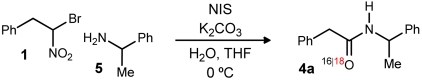
| Entry* | NO2 label (% N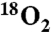 ) ) |
H2O (%18O) | Atmosphere | Amide (% 18O) | Δ18O | Yield† (%) |
| 1‡ | 0 | > 99 | open | < 1 | < 1 | 75 |
| 2‡ | 82 | 0 | open | 17 | −65 | 76 |
| 3§ | 82 | 0 | open | 49 | −33 | 70 |
| 4§ | 82 | > 99 | open | 49 | −33 | 70 |
| 5§ | 82 | 0 | Ar | 66 | −16 | 70 |
| 6 | 0 | 0 |
 ¶ ¶
|
83 | +83 | 68 |
*Reactions employed one equivalent of α-bromo nitroalkane (0.2 M in THF), with N-iodosuccinimide (one equivalent) added as the final reagent at 0 °C. “Open” atmosphere refers to use of a static atmosphere provided by a cap or septum. Other variations used a balloon of the indicated gas. Isotopic distribution determined by high resolution mass spectrometry.
†Isolated yields.
‡1.2 equivalents of amine used.
§Five equivalents of amine used to replace potassium carbonate base.
¶97% enriched 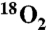 gas used.
gas used.
