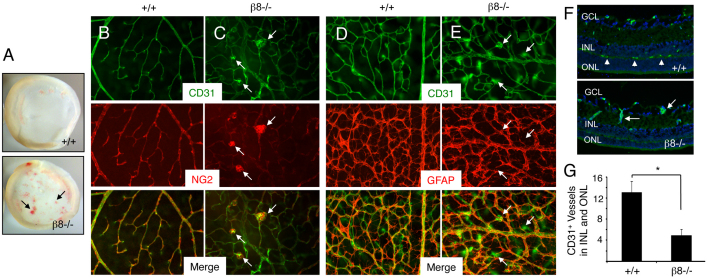
Fig. 3.
Impaired development of the secondary retinal vascular plexus in β8–/– mice. (A) Images of whole retinas isolated from P14 wild-type (upper panel) or β8–/– (lower panel) littermates. Note the severe intraretinal hemorrhage in the β8–/– sample (arrows). (B,C) Retinas from P14 wild-type (B) or β8–/– (C) littermates were labeled with anti-CD31 and anti-NG2 antibodies to reveal vascular endothelial cells and pericytes, respectively. Note that in β8–/– mice vascular endothelial cells display impaired sprouting into deeper retinal layers and form glomeruloid-like tufts that contain NG2-positive pericytes (arrows in C). (D,E) Retinas from P14 wild-type (D) or β8–/– (E) littermates were labeled with anti-CD31 and anti-GFAP antibodies to reveal vascular endothelial cells and astrocytes, respectively. Although β8–/– mice blood vessels form abnormal glomeruloid-like structures, the GFAP-expressing astrocyte network underlying the primary plexus appears normal (arrows in E). (F) Coronal sections through wild-type (upper panel) and β8–/– (lower panel) retinas were stained with anti-CD31 to reveal vascular endothelial cells. Unlike the elaborate vascular sprouts in wild-type samples (arrowheads in upper panel), note the diminished blood vessel sprouting into the outer nuclear layer (ONL) and inner nuclear layer (INL) in β8–/– mice (arrows in lower panel). GCL, ganglion cell layer. (G) Quantification of integrin-dependent formation of the secondary vascular plexus determined by quantifying blood vessels in the INL and ONL of wild-type and β8–/– mice (n=3 sections per genotype). *P<0.001. Error bars represent s.d. Images in B-F are shown at 200× magnification.