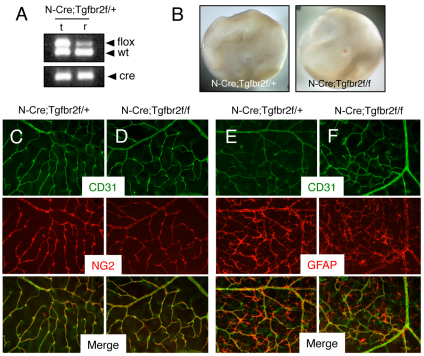
Fig. 6.
Retinal angiogenesis is not perturbed in mice lacking Tgfbr2 gene expression in astrocytes. (A) Nestin-Cre-mediated deletion of the Tgfbr2flox allele in genomic DNA isolated from the retina (r) but not matched tail snips (t) as confirmed by PCR analysis. In the retina there is a reduction in the ∼500 bp band owing to Cre-mediated recombination of the Tgfbr2flox allele. (B) Images of retinas isolated from P14 Nestin-Cre;Tgfbr2flox/+ control (left panel) or Nestin-Cre;Tgfbr2flox/flox mutant mice (right panel). Note the absence of hemorrhage in the mutant retinas. (C,D) Retinas from P14 control (C) and Nestin-Cre;Tgfbr2flox/flox mutant (D) mice were immunolabeled with anti-CD31 and anti-NG2 antibodies to visualize vascular endothelial cells and pericytes, respectively. Note the normal blood vessel cytoarchitecture containing vascular endothelial cells and pericytes in control and mutant samples. (E,F) Retinas from P14 control (E) and Nestin-Cre;Tgfbr2flox/flox mutant (F) mice were immunolabeled with anti-CD31 and anti-GFAP antibodies to visualize vascular endothelial cells and astrocytes, respectively. Note the normal endothelial cell and astrocyte interactions in both control and mutant samples. Images in C-F are shown at 200× magnification.