Abstract
Recent observations have demonstrated that nanomaterials may be toxic to human tissue. While the ability of nano-scaled particulate matter is known to cause a range of problems in respiratory system, recent observations suggest that the nervous system may be vulnerable as well. In the current paper we asked whether exposure of primary neuronal cell cultures to nanoparticles might compromise regenerative axon growth. Regenerative response was triggered by performing a conditioning lesion of sciatic nerve five days prior to collection of dorsal root ganglia (DRG). DRG neurons were plated at a low density and incubated with multi-walled carbon nanotubes (MWCNT) (0.1 – 10 μg/ml in 10% of surfactant in saline) overnight. The experiments showed that exposure of DRG cultures to MWCNT significantly impaired regenerative axonogenesis without concomitant cell death. These results indicate that MWNCTs may have detrimental effect on nerve regeneration and may potentially trigger axonal pathology.
Keywords: nanoparticles, multi-walled carbon nanotubes, regenerative axon growth, dorsal root ganglion
Introduction
Nanoparticles (NP) are small objects with at least one dimension less than 100 nanometers and exhibit an extremely high surface to volume ratio [16]. These unique properties of NP account for their diverse physiochemical interactions with biologics and potential to contribute to medical and environmental dangers [1, 39]. In particular, investigation of multiwall carbon nanotubes (MWCNTs) toxicity is critically important, because of MWCNTs’ increasing use in nanotechnology, electronics, optics, as well as in medicine (reviewed in [34]). MWCNTs are allotropes of carbon with a cylindrical nanostructure. These cylindrical structures have novel properties that make them useful in many medical applications including drug delivery, biosensors and scaffolds. Their final usage, however, may be limited by their potential toxicity.
Recent observations have demonstrated that due to their size, NP may exhibit greater toxicity to human tissue and cell cultures, resulting in increased oxidative stress [19, 35], inflammatory cytokine production and cell death [9]. NP are reported to cross biological membranes [5], and gain access to the blood stream following inhalation or ingestion [13, 27], or through the skin [14]. Once in the blood stream, NP can be transported throughout the body and be detected in various organs and tissues [28, 29], including heart, kidney, liver, and brain [6, 7, 10, 13, 26]. In particular, it was shown that some NP can penetrate the blood-brain barrier, affecting brain signaling linked to Alzheimer’s and Parkinson’s diseases, and decrease of cognitive function [5, 19, 31, 32]. However, the mechanism of nanomaterials’ toxicity on the nervous system has been poorly investigated (reviewed in [12]). In the current report we asked whether exposure of primary neuronal cell cultures to MWCNTs might compromise regenerative axon growth. We explored the effects of exposure of dissociated dorsal root ganglia (DRG) cultures to MWCNT on regenerative axon growth as well as cellular viability. Our data demonstrated that MWCNT exposure exerted dose-dependent biological effects on regenerative axonogenesis in DRG neuronal cells.
Materials and Methods
Animals and surgery
Experiments were performed on 8-wk-old CD-1 mice (Charles River laboratories) according to the guidelines of the animal care and use committee of East Carolina University, an AAALAC-accredited facility. To induce regenerative growth of axon, a conditioning crush lesion of peripheral axons of dorsal root ganglion was made on right side of sciatic nerve of each animal. Under ketamine (18/mg/ml)-xylazine (2mg/ml) anaesthesia, the sciatic nerve was crushed in the mid-thigh for 15 seconds with fine hemostat, following the routine lab protocol [22].
Cell culture and the treatment of MWCNT
Primary cultures of conditioned sensory neurons from DRG were performed as described previously [33]. Briefly, 5 days before DRG collection, conditioning lesions were performed on the sciatic nerve at the level of mid-thigh for each mouse. DRGs (from L4 and L5) were then collected into media (DMEM/F12 + N2+ Glutamine+ 10% horse serum +Penicillin/ Streptomycin), briefly rinsed, and minced with microscissors. Conditioned DRGs were dissociated with 500 U/ml collagenase (Sigma, St. Louis, MO) and 0.05% trypsin-EDTA (Invitrogen, Grand Island, NY). Cells were resuspended and plated at low density on poly-l-ornithine/laminin coated cover slips. MWCNTs (C-grade) were prepared in 10% surfactant /sterile saline at the concentration of 1mg/ml. The mixture was bath-sonicated (Sonicator S-4000, Misonix Ultrasonic Liquid processor) for 4 minutes to obtain a suspension. The stock was kept in refrigerator at 4°C for no more than 2 weeks. Final concentration of 0.1μg/ml, 1μg/ml, 5μg/ml, and 10μg/ml were applied to DRG cultures and incubated overnight.
Generation of MWNCT
MWCNTs were grown by NanoTech Labs Inc. (Yadkinville, NC), to approximately 25 nm in width and 10–20 μm in length using the chemical vapor deposition (CVD) process. Iron was used as the single metal catalyst. The elemental composition as determined by thermogravimetric analysis (TGA) revealed that the MWCNT samples contained < 5% Fe by weight. A detailed characterization of these MWCNT and the material in suspension were reported in previous publication [36]. All materials were generated in a sterile environment and had been tested and determined to be free of endotoxin contamination. Prior to their use in biological assays, the MWCNTs were resuspended in a sterile saline with 10% surfactant (InfasurfR with a concentration of 35 mg/ml phospholipids, gift from Forest Pharmaceuticals, Inc).
Morphological assessment of regenerative axon growth
Cover slips with adherent cells were rinsed with PBS, fixed with 4% paraformaldehyde and immunostained, as described previously [23]. To visualize neurons and their processes, cells were incubated with primary mouse anti-TUJ1 (Covance, Princeton, NJ) antibody at 1:100 dilution overnight at 4°C and with anti-mouse FITC secondary antibodies for 1 h at room temperature. Ten to thirty individual images were taken from each cover slip at 40× magnification using Olympus IX81 motorized inverted microscope [23]. Neuron processes were traced if they were more than one cell body in length, and secondary and higher order branches of the neurite were scored after a branch point (NIH ImageJ). All experiments were performed in triplicate.
Detection of cellular apoptosis
The effect of MWCNT on apoptosis was detected by TUNEL staining with In Situ Cell Death Detection Kit (Roche Applied Science, 68298 Mannheim, Germany). DRG cultures were fixed with 4% paraformaldehyde in PBS rinsed in permeabilization solution (0.1% Trition X-100 in 0.1% sodium citrate) for 2 min on ice, and incubated in a humidified atmosphere for 1 h at 37°C with the TUNEL reaction mixture. A negative control and a positive control were included in each staining series. Negative control was incubated with label solution instead of the TUNEL reaction mixture. Positive control was treated with recombinant DNase I (200U/ml) for 10 min at room temperature prior to the incubation with the TUNEL reaction mixture.
Statistical analysis
Data were expressed as mean ± SEM and analysed using GraphPad Prism version 5 for Windows (GraphPad Software; San Diego, CA, USA). One-way ANOVA with post-hoc Tukey-type multiple comparison test or t-test were used to identify the difference between means. The level of significance was set at p < 0.05.
Results and Discussion
The morphological effects of MWCNT exposure on primary neuronal cells
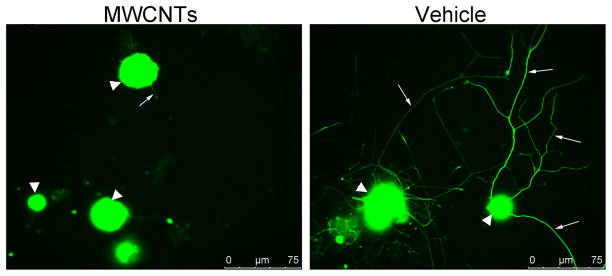
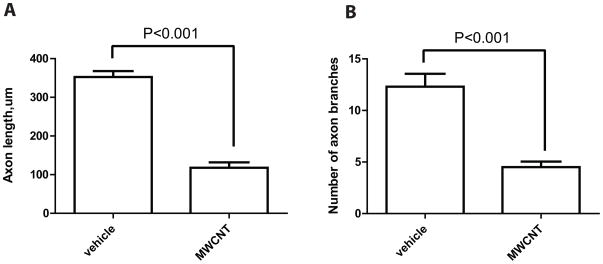
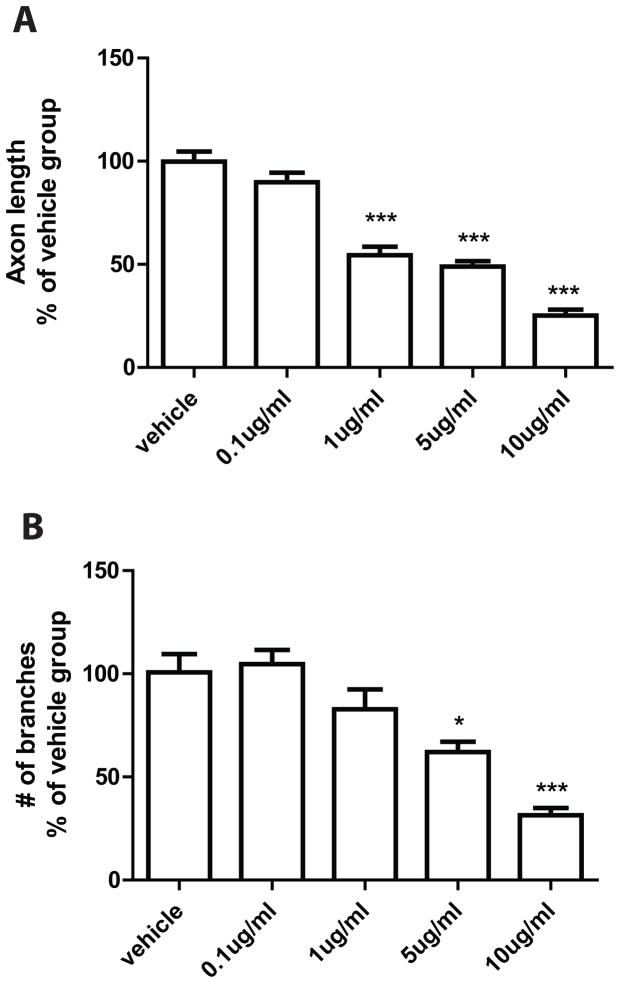
The axon regeneration response was measured in vitro following a “conditioning” sciatic nerve crush, which results in rapid axon elongation over 24–48 h in vitro [30, 37]. DRG neuronal cultures were examined 24 h after plating cells onto pre-coated glass cover slips. The analysis revealed a significant difference between cells incubated with MWCNTs (10 μg/ml in 10% of surfactant in saline) and cells incubated with the vehicle alone (10% of surfactant in saline). DRG neurons incubated with MWCNTs exhibited stunted neurite outgrowth with fewer processes and branches (Fig. 1). Quantification of mean neurite lengths revealed a significant decrease in neurite length (69 ± 10μm, n=13, P<0.001) associated with MWCNTs exposure in comparison to vehicle-treated cultures (330 ± 23μm, n=18) (Fig. 2). Additionally, incubation with MWCNTs at the highest dose of 10 μg/ml produced a striking reduction of neurite branching (Figure 1). Quantitation of the number of branches revealed significant reduction in the mean number of branches per cell between MWCNTs-treated (2.1 ± 0.43, n=13) and vehicle-treated groups (11 ± 1.9, n=16) (Figure 2). We also examined if there was a dose-dependent effect of MWCNT on neurite outgrowth at lower doses. Cells cultures were incubated for 24 hours with either 0.1μg/ml, 1μg/ml, or 5μg/ml of MWCNT and were analyzed for the same morphological end points. The lowest applied dose (0.1μg /ml) did not significantly inhibit growth of neurites while doses 1, 5 and 10 μg/ml revealed a dose-dependent compromise of regenerative axon growth both in length and extent of branching (Figure 3). Together, these data indicated a significant dose-dependent impairment of regenerative axon growth following MWCNT exposure.
Figure 1. Indirect immunofluorescence of DRG neurons 24 hours after plating.
Indirect immunofluorescence was performed against neuronal β-tubulin using primary mouse anti-TUJ1 antibody, and anti-mouse FITC secondary antibodies. Left panel shows DRG neurons incubated overnight with 10ug/ml of MWCNTs in 10% of surfactant in saline. Right panel shows control DRG neurons incubated with vehicle (10% of surfactant in saline). Big white arrowheads point at DRG neurons. Small arrows point at axons.
Figure 2. Decrease of axon length and brunching in DRG cultures after exposure to MWCNTs for 24 hours.
A: Mean axon length (μm) calculated after tracing neuritis with ImageJ (NIH) software. B: Mean number of branches. Processes initiated from the cell body were scored if they extended greater than 1 cell body in length from the neurite, and secondary and higher order branches of the neurite were scored after a branch point.
Figure 3. Dose-dependent responses of DRG axon outgrowth to MWCNT treatment.
The statistical analyses were performed on the length (A) and the branch number (B) of the axons in dissociated DRG cultures after overnight incubation with vehicle, 0.1μg/ml, 1μg/ml, 5μg/ml and 10 μg/ml of MWCNT. The inhibition of regenerative axon growth is related to the dose of MWCNT. ***- P<0.001
The effects of MWCNT exposure on viability of DRG neuronal cells
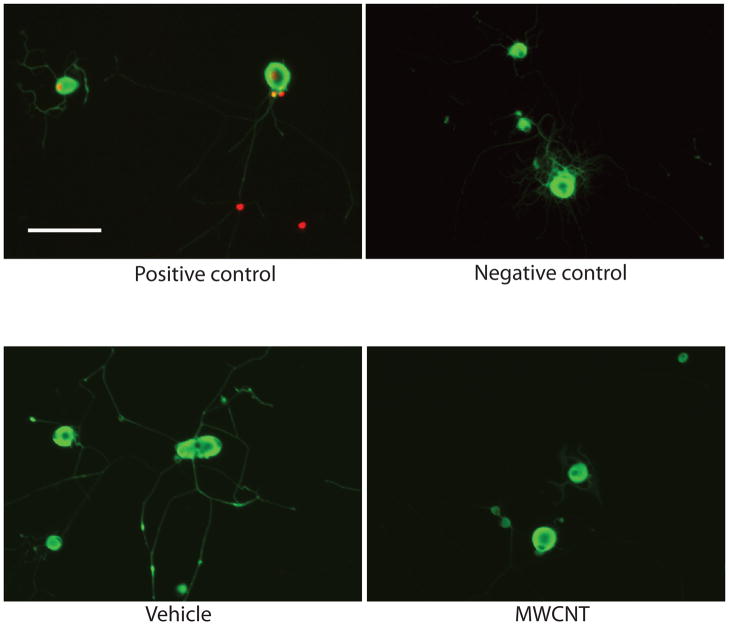
Interestingly, MWCNTs, while inhibiting neurite outgrowth, did not induce cell death. The Tunnel assay showed no notable differences in cell death between control and MWCNTs treated cultures (Figure 4). These data indicated that even at the highest dosage (10 μg/ml), MWCNT treatment did not resulted in apoptosis of DRG neurons. MWCNTs have recently appeared as a new choice for various biomedical applications including cancer treatment, drug delivery and bioengineering. Their potential in nanomedicine though may be limited because of potential toxicologic implications. The lack of extensive evaluation of cytotoxicity with non-functionalized MWCNTs, restricts predictability and limits comparison between research results [11]. Even less studied are the effects of MWCNTs on neuronal cells. To address this problem, we investigated response of primary neuronal culture to MWCNTs exposure. Our results illustrated an interesting interaction between MWCNTs and regenerative axon growth in DRG cultures. We demonstrated that when primary neuronal cultures were treated with MWCNT at different concentrations (0.1 – 10 μg/ml), the nerve cells exhibited a dose-depended deficiency in their regenerative ability. Interestingly, the treatment with MWCNT inhibited the elongation and branching of axons, but did not induce apoptosis of those cells. Other studies, in non-neuronal cells, reported dose-dependent cytotoxicity as a decrease in cell number and increase in apoptosis [8, 25].
Figure 4. Effect of MWCNT on cell apoptosis.
TUNEL technique was used to label DNA strand breaks induced apoptosis. No damaged DNA was detected in vehicle treated or MWCNT treated DRG neuronal cell cultures. The images captured under microscopes from both vehicle treated group and MWCNT treated group were similar to the images from negative control group, which indicated the exposure of MWCNT at dose of 10 μg/ml overnight didn’t increase cell death of DRG neuronal cells. (Scale bar=100 μm).
Similar to our observation, a previous report showed that when cultured on unmodified MWCNTs, hippocampal neurons extended only one or two neuritis and exhibited very few branches [21]. In contrast, functionalized carbon nanotubes were explored for their beneficial use in nerve regeneration. Neurons grown on MWCNTs coated with the bioactive molecule 4-hydroxynonenal exhibited extensive sprouting [21]. Low concentrations (0.11–1.7 μg/ml) of functionalized MWCNTs, modified by amino groups, actually promoted outgrowth of chick embryo DRG neurons and rat PC12 cells [20]. In addition, in vivo findings recently showed that pre-treating rats with amine-modified single-walled carbon nanotubes could protect neurons and enhance the recovery of behavioural functions in an induced stroke model [15]. However, while it is widely accepted that the toxicity of MWCNT is strongly affected by their surface modifications [11], it is not clear if bio-coating may protect from MWCNTs’ potential toxicity in long term.
Possible mechanism of interactions between neurons and MWCNT
There are several possible mechanisms, which could explain the effect of MWCNT on nervous system. In the first, the cytotoxic effects are resulted from the direct interaction between the NP and neuronal cells. MWCNT could be translocated in nervous system by retrograde axonal transport through olfactory nerve ending in the nasal cavity [10] or other sensory nerve fibers that project into pulmonary system [13]. A second mechanism may be associated with the induction of an extra pulmonary transport response. Inhaled NPs are thought to enter the pulmonary vasculature and lymphatic’s, eventually entering the systemic circulation only to deposit in a variety of organs [24]. Several observations have demonstrated the increase in authophagic activity upon exposure of cells to certain nanomaterials, resulting in increased autophagic vacuoles formation and the disruption of autophagy pathway [31]. Other studies revealed that MWCNT could mechanically penetrate the cytoplasm and further penetrate subcellular structures, including nuclei [4]. In this later scenario, NPs could change the normal function of ion channels within neurons. Since the ion channels play a crucial role in a broad range of biological processes in neuron, alteration or impairment in cell function would be expected [38]. Moreover, a new observation has demonstrated the effect of carbon nanotubes on the electrical activity of isolated neurons [3]. Using single-cell electrophysiology, the investigators showed that hippocampal neurons grown on carbon nanotube substrate exhibited significantly enhanced backpropagation of the action potential to dendrites. The authors further proposed that nanotubes improve the responsiveness of neurons by forming tight contacts with the cell membranes that might favor electrical shortcuts between the proximal and distal compartments of the neuron.
The adverse effect of MWCNT exposure on regenerative axon growth can also be explained by systemic effects. Several studies have investigated the involvement of neuroinflammatory and oxidative stress in the neurotoxic effect of carbon-based or metal-based NPs [13]. Mice with the aspiration of MWCNT had significantly increased level of cytokines in the blood [10]. Single dose of MWCNT by pharyngeal aspiration was sufficient to induce the expression of brain-region-specific mRNAs of chemokines, cytokines, and markers of cellular stress [13]. Iron- and aluminum-sulfate treatment in primary neural cells culture triggered the production of reactive oxygen species (ROS) and induced the expression of specific micro RNAs that were up-regulated in Alzheimer’s disease brains [18]. Studies also showed that administration of NPs in mice can trigger oxidative stress response and interfere with mitochondrial function in the brain [17]. Inflammation and ROS are known regulators of neurite growth and branching in other models [2]. Therefore, an increase in inflammation and oxidative stress may partially explain the impairment in regenerative axon growth following MWCNT exposure.
While several findings established the feasibility of using nanotubes as substrates for nerve cell growth, our current data suggest caution, as the neurons compromised by “conditioning lesion” are sensitive to MWCNTs exposure, showing compromised regenerative response.
Further studies focused on specific molecular pathways that mediate action of MWCNTs should yield important insights into mechanisms that may provide the means for therapeutic targeting of nervous system.
Conclusions
The application of nanomaterials into new products has dramatically increased in areas of industry, medicine and research. Thus, a complete understanding of the interaction between nanoparticles and target cells is urgently required. However, data on both positive functionality and negative potential hazard of MWCNT exposure are rare.
Our results indicate that exposure to MWNCTs may have detrimental effect on regenerative axon growth and may potentially trigger axonal pathology. These results necessitate further studies related to the functionality and toxicity of nanostructures in neuronal cells.
Highlights.
Multi-walled carbon nanotubes (MWCNT) inhibited regenerative axon growth in dorsal root ganglia cultures
MWCNT exposure decreased axon length and axon branching, without cell death
MWNCTs may have detrimental effect on regenerative axon growth and may potentially trigger axonal pathology.
Acknowledgments
This work was supported in part by East Carolina University Research Developmental Award to AKM and by NIEHS grant 1R15ES019760-01 to AKM and NIH RO1 ES06008 (CJW).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DW wrote the manuscript and performed cell culture, morphological assessment, and apoptosis assay. EP carried out the cell culture studies, immunofluorscence, and performed the statistical analysis. CW participated in designing the study and revising the manuscript. AM conceived the study, and participated in its design and coordination and helped to draft and revise the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Di Wu, Email: WUD07@students.ecu.edu.
Elena S. Pak, Email: pake@ecu.edu.
Christopher J. Wingard, Email: wingardc@ecu.edu.
Alexander K. Murashov, Email: murashoval@ecu.edu.
References
- 1.Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM. Nanoparticles for detection and diagnosis. Adv Drug Deliv Rev. 2010;62:316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth BM, Gustafson SJ, Kuhn TB. Neutral sphingomyelinase activation precedes NADPH oxidase-dependent damage in neurons exposed to the proinflammatory cytokine tumor necrosis factor-alpha. Journal of neuroscience research. 2011 doi: 10.1002/jnr.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, Gambazzi L, Markram H, Grandolfo M, Scaini D, Gelain F, Casalis L, Prato M, Giugliano M, Ballerini L. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nature nanotechnology. 2009;4:126–133. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 4.Cheng C, Muller KH, Koziol KK, Skepper JN, Midgley PA, Welland ME, Porter AE. Toxicity and imaging of multi-walled carbon nanotubes in human macrophage cells. Biomaterials. 2009;30:4152–4160. doi: 10.1016/j.biomaterials.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Chopra D, Gulati M, Saluja V, Pathak P, Bansal P. Brain permeable nanoparticles. Recent Pat CNS Drug Discov. 2008;3:216–225. doi: 10.2174/157488908786242461. [DOI] [PubMed] [Google Scholar]
- 6.Cozzi E, Hazarika S, Stallings HW, 3rd, Cascio WE, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2006;291:H894–903. doi: 10.1152/ajpheart.01362.2005. [DOI] [PubMed] [Google Scholar]
- 7.Cozzi E, Wingard CJ, Cascio WE, Devlin RB, Miles JJ, Bofferding AR, Lust RM, Van Scott MR, Henriksen RA. Effect of ambient particulate matter exposure on hemostasis. Transl Res. 2007;149:324–332. doi: 10.1016/j.trsl.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP, Cooke PA, Gray JW, Chen FF. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5:2448–2464. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 10.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdorster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firme CP, 3rd, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine : nanotechnology, biology, and medicine. 2010;6:245–256. doi: 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Hu YL, Gao JQ. Potential neurotoxicity of nanoparticles. Int J Pharm. 2010;394:115–121. doi: 10.1016/j.ijpharm.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Hubbs AF, Mercer RR, Benkovic SA, Harkema J, Sriram K, Schwegler-Berry D, Goravanahally MP, Nurkiewicz TR, Castranova V, Sargent LM. Nanotoxicology--a pathologist’s perspective. Toxicol Pathol. 2011;39:301–324. doi: 10.1177/0192623310390705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larese FF, D’Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, Maina G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology. 2009;255:33–37. doi: 10.1016/j.tox.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Park J, Yoon OJ, Kim HW, Lee do Y, Kim do H, Lee WB, Lee NE, Bonventre JV, Kim SS. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol. 2011;6:121–125. doi: 10.1038/nnano.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkov I, Satterstrom FK, Corey LM. Nanotoxicology and nanomedicine: making hard decisions. Nanomedicine. 2008;4:167–171. doi: 10.1016/j.nano.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40:4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 18.Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. Journal of inorganic biochemistry. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto K, Sato C, Naka Y, Whitby R, Shimizu N. Stimulation of neuronal neurite outgrowth using functionalized carbon nanotubes. Nanotechnology. 2010;21:115101. doi: 10.1088/0957-4484/21/11/115101. [DOI] [PubMed] [Google Scholar]
- 21.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000;14:175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 22.McMurray R, Islamov R, Murashov AK. Raloxifene analog LY117018 enhances the regeneration of sciatic nerve in ovariectomized female mice. Brain Res. 2003;980:140–145. doi: 10.1016/s0006-8993(03)02984-6. [DOI] [PubMed] [Google Scholar]
- 23.Murashov AK, Pak ES, Hendricks WA, Tatko LM. 17beta-Estradiol enhances neuronal differentiation of mouse embryonic stem cells. FEBS Lett. 2004;569:165–168. doi: 10.1016/j.febslet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 25.Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn Dis. 2010;20:S1-65–72. [PMC free article] [PubMed] [Google Scholar]
- 26.Praetorius M, Brunner C, Lehnert B, Klingmann C, Schmidt H, Staecker H, Schick B. Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol. 2007;127:486–490. doi: 10.1080/00016480600895102. [DOI] [PubMed] [Google Scholar]
- 27.Rothen-Rutishauser B, Blank F, Muhlfeld C, Gehr P. In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin Drug Metab Toxicol. 2008;4:1075–1089. doi: 10.1517/17425255.4.8.1075. [DOI] [PubMed] [Google Scholar]
- 28.Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. 2006;34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 29.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Snider WD, Zhou FQ, Zhong J, Markus A. Signaling the pathway to regeneration. Neuron. 2002;35:13–16. doi: 10.1016/s0896-6273(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 31.Stern ST, Johnson DN. Role for nanomaterial-autophagy interaction in neurodegenerative disease. Autophagy. 2008;4:1097–1100. doi: 10.4161/auto.7142. [DOI] [PubMed] [Google Scholar]
- 32.Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. Association of black carbon with cognition among children in a prospective birth cohort study. Am J Epidemiol. 2008;167:280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- 33.Twiss JL, Smith DS, Chang B, Shooter EM. Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol Dis. 2000;7:416–428. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- 34.Veetil JV, Ye K. Tailored carbon nanotubes for tissue engineering applications. Biotechnol Prog. 2009;25:709–721. doi: 10.1002/bp.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol In Vitro. 2009 doi: 10.1016/j.tiv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Particle and fibre toxicology. 2011;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad Z, Ren G. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface. 2010;7(Suppl 4):S411–422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]