Figure 3.
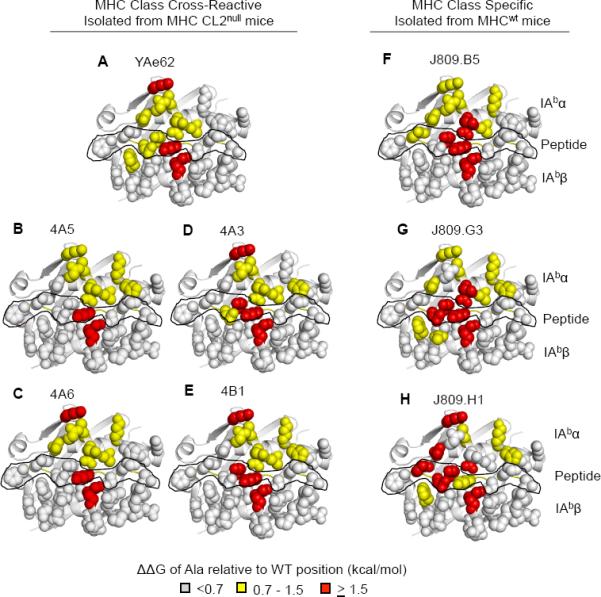
Energetic “Footprint” of MHC cross-reactive TCRs and MHC specific TCRs.
(A–E) MHC side chain contribution for binding relative to wild type IAb-3K for the (A) YAe62 TCR, (B,C) 4A5, 4A6 (Vα11+ TCRs), (D–E) 4A3, 4B1 (Vα5+ TCRs) isolated from MHC CL2null YAe62β mice.
(F–H) MHC side chain contribution of binding relative to wild type IAb-3K for the J809.B5, J809.G3, or J809.H1, (Vα2+ TCRs) isolated from MHCwt YAe62β mice.
Individual IAb-3K alanine substitutions covering the potential TCR binding surface were made in the IAbα chain, IAbβ chain and 3K peptide (see Figure S5). ΔΔG >1.5 kcal/mol are colored red, ΔΔG = 0.7–1.5 kcal/mol colored yellow, ΔΔG <0.7 kcal/mol are colored light gray. The peptide residues are outlined in black. Data are averages of three independent measurements.
