Table 2.
Functionalizaation of tetrahydroisoquinolines enabled by visible light-mediated photoredox catalysis
| entry | substrate | product | yielda |
|---|---|---|---|
 |
 |
||
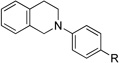
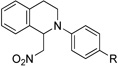
| 1b | 1 R = H | 4 R = H | 95 |
| 2b | 3 R = Br | 5 R = Br | 93 |
 |
 |
||
| 3b | 6 | 7 | 95 (d.r.= 2:1) |
 |
 |
||
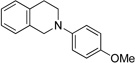
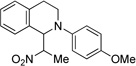
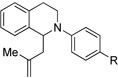
| 4c | 1 R = H | 8 R = H | 85 |
| 5c | 6 R = OMe | 9 R = OMe | 44 |
 |
 |
||
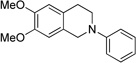
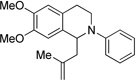
| 6c | 10 | 11 | 43 |
 |
 |
||
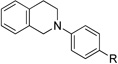
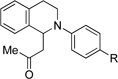
| 7d | 1 R = H | 12 R = H | 59 |
| 8d | 3 R = Br | 13 R = Br | 65 |
 |
 |
||
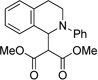
| 9e | 1 | 14 | 69 |
 |
 |
||
| 10e | 1 | 15 | 68 (d.r.= 3:2) |
Isolated percent yields after chromatography on SiO2.
Ru(bpy)3Cl2 (1 mol %), BrCCl3 (3 equiv), DMF, blue LEDs, 3 h, then no light, Et3N (5 equiv), nitroalkane (5 equiv).
Ru(bpy)3Cl2 (1 mol %), BrCCl3 (3 equiv), DMF, blue LEDs, 3 h, then no light, methallyl trimethylsilane (5 equiv).
Ru(bpy)3Cl2 (1 mol %), BrCCl3 (3 equiv), DMF, blue LEDs, 3 h, then no light, Et3N (5 equiv), silyl enol ether (5 equiv).
Ru(bpy)3Cl2 (1 mol %), BrCCl3 (3 equiv), DMF, blue LEDs, 3 h, then no light, Et3N (5 equiv), 1,3-dicarbonyl (5 equiv).
