Figure 2.
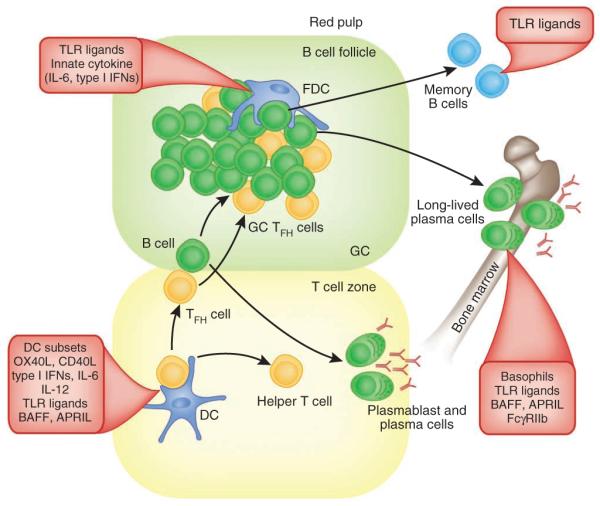
Programming antibody responses with innate immunity. Antigen-specific antibody responses to T cell–dependent antigens develop along two anatomically and functionally distinct pathways132–135. DC-mediated stimulation of antigen-specific TH cells in the T cell–rich areas is regulated by the DC subset and the PRRs triggered. Activated antigen-specific B cells migrate to the interface between the B cell follicle and T cell area132–135. Here, they interact with helper T cells, which results in the clonal expansion of B cells; these migrate to the bridging channels at the edges of the lymphoid areas of the spleen or the medullary cords in the lymph nodes and differentiate into short-lived plasma cells132–135. Other activated B cells migrate into B cell follicles and proliferate rapidly and form germinal centers (GC). In addition, some helper T cells (TFH cells) express CXCR5 and migrate into the follicles. Induction of TFH cells is controlled by the nature of the DC subset, innate cytokines, TLR ligands, costimulatory molecules, TLR ligands and so on. In the early phase of GC development, the dividing B cells (centroblasts) downregulate cell surface expression of immunoglobulin and undergo somatic hypermutation of their immunoglobulin genes132–135. Then, the centroblasts cease to divide and re-express their mutated immunoglobulin receptor, and cells (centrocytes) with heightened affinity for the antigen are thought to be selected for enhanced affinity of binding to antigen-antibody complexes on the follicular DCs, as well as by helper T cells in the light zone136. Positively selected centrocytes differentiate into long-lived plasma cells that migrate to the bone marrow or differentiate into recirculating memory B cells. The survival of long lived plasma cells or memory B cells is controlled by innate parameters such as TLR ligands, basophils14, BAFF or APRIL.
