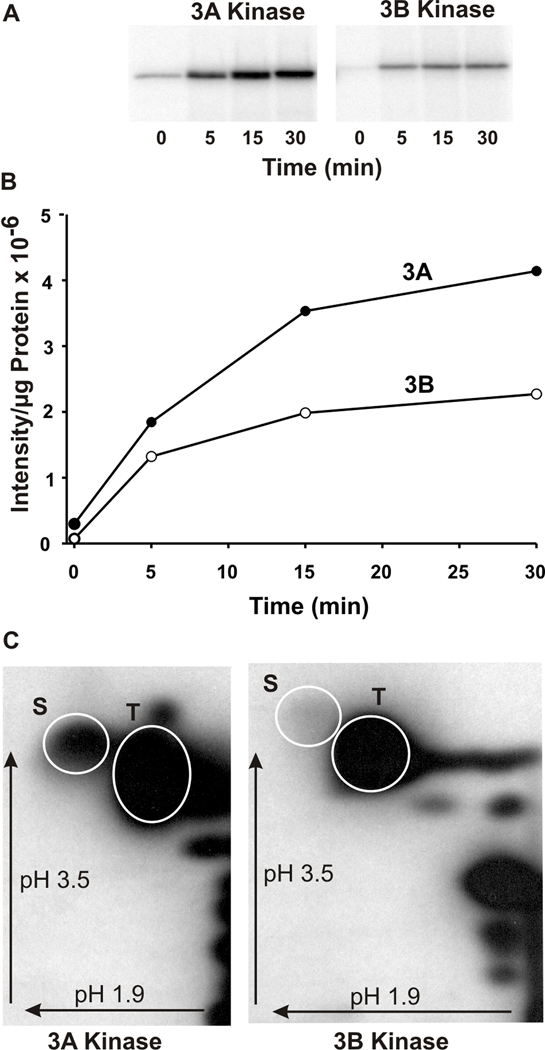
Figure 2. The kinase/transition domains of mMyo3A and 3B autophosphorylate on serine and threonine residues.
A. Phosphorimages of the autophosphorylation of mMyo3A and 3B with time. B. Plot of the intensities of the phosphorylated bands shown in A versus time per µg of protein incubated. Incubations contained 5.6µg mMyo3A (closed circles) and 2.4µg mMyo3B (open circles). C. Phosphorimages of phosphorylated amino acids in the kinase/transition domains of mMyo3A and 3B following a 1hr incubation in vitro. Incubations contained 10µmol/l and 8µmol/l of mMyo3A and 3B, respectively. Phosphorylated amino acids were separated by two dimensional high voltage thin layer electrophoresis using a pH 1.9 buffer in the first dimension and a pH 3.5 buffer in the second dimension. Arrows indicate the directions of electrophoretic migration. White circles show the locations of the phosphoserine (S) and phosphothreonine (T) amino acid standards.